靶向共享β-连环蛋白突变的tcr工程T细胞可根除实体肿瘤
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
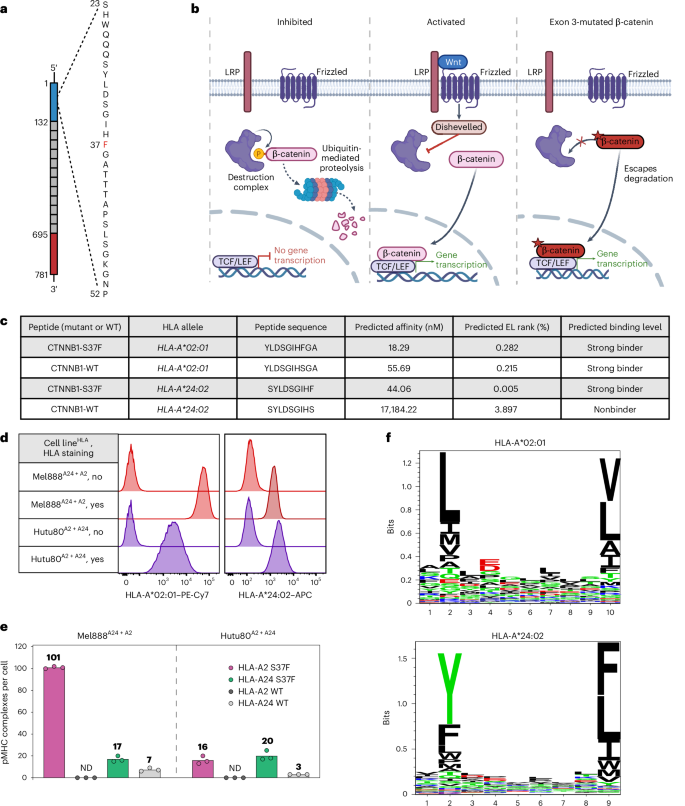
由复发性驱动突变编码的hla结合肽是T细胞定向免疫治疗的候选靶点。在此,我们在自然表达突变和HLA等位基因的细胞系中发现了两个由CTNNB1S37F突变编码的新肽,这些突变存在于HLA- a *02:01和HLA- a *24:02分子中。这种突变导致β-连环蛋白的功能增加,据估计,在美国每年有7000例新的癌症病例发生这种突变。特异性识别突变肽的T细胞受体(TCRs)是从原始健康供体T细胞中分离出来的。用CTNNB1-S37F TCRs重定向的T细胞在体外有效地杀死CTNNB1S37F+细胞系和患者来源的类器官,并在黑色素瘤细胞系小鼠模型和自然表达突变和限制性HLA的子宫内膜腺癌患者来源的异种移植模型中根除已建立的肿瘤。我们提出靶向CTNNB1-S37F的TCR-T细胞可以作为实体癌免疫治疗的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。

TCR-engineered T cells targeting a shared β-catenin mutation eradicate solid tumors
HLA-bound peptides encoded by recurrent driver mutations are candidate targets for T cell-directed immunotherapy. Here we identify two neopeptides encoded by the CTNNB1S37F mutation presented on the frequent HLA-A*02:01 and HLA-A*24:02 molecules in cell lines naturally expressing the mutation and HLA alleles. This mutation leads to a gain of function in β-catenin and is estimated to occur in >7,000 new cancer cases annually in the United States. T cell receptors (TCRs) that specifically recognize the mutant peptides were isolated from naive healthy donor T cells. T cells redirected with CTNNB1-S37F TCRs efficiently killed CTNNB1S37F+ cell lines and patient-derived organoids in vitro and eradicated established tumors in a melanoma cell line mouse model and a patient-derived xenograft model of endometrial adenocarcinoma naturally expressing the mutation and the restricting HLA. We propose that TCR-T cells targeting CTNNB1-S37F can serve as a basis for solid cancer immunotherapy. TCR-T cells are T cells engineered to express a specific T cell receptor. Here the authors present a TCR-T cell that targets CTNNB1-S37F, corresponding to a shared cancer driver mutation. This immunotherapy killed solid tumors when applied to a patient-derived xenograft model in mice.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


