小隐孢子虫多药耐药蛋白赋予对有毒肠道微生物代谢物的抗性
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
小隐孢子虫亚型的致病性不同,但潜在的因素在很大程度上是未知的。我们发现两种基因相似的小孢子虫分离株在体外生长得同样好,但在免疫功能低下的小鼠中致病性不同。通过抗生素治疗,无毒性菌株的卵囊脱落减少,表明微生物群赋予的定植抗性易感性。这种抗性与一种编码寄生虫ABC转运蛋白的基因有关,并增强了传染性。分子分析表明,ABC转运蛋白属于多药耐药蛋白(MRP)家族。CpMRP1结合细菌代谢物,特别是抑制小芽胞杆菌生长的脱氧胆酸(DCA)。CpMRP1从小颗粒出口到寄生虫-宿主界面,可能介导外源性药物的出口。CpMRP1的缺失降低了小鼠的传染性和DCA抗性,CpMRP1的多态性决定了对DCA的易感性。这些结果将CpMRP1定义为小孢子虫对微生物组介导的抑制的敏感性的决定因素,从而影响传染性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
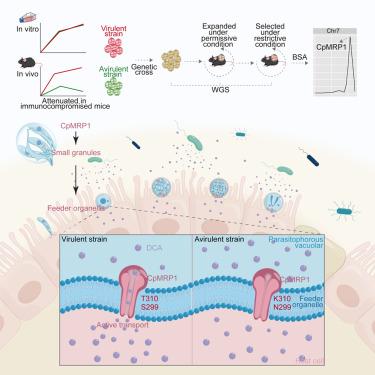
Cryptosporidium parvum multidrug resistance protein confers resistance to toxic gut microbial metabolite
Cryptosporidium parvum subtypes differ in pathogenicity, but the underlying factors are largely unknown. We show that two genetically similar C. parvum isolates grow equally well in vitro but differ in pathogenicity in immunocompromised mice. Reduced oocyst shedding of the avirulent strain was restored by antibiotic treatment, suggesting susceptibility to colonization resistance imparted by the microbiota. This resistance was associated with a gene encoding a parasite ABC transporter and enhanced infectivity. Molecular analyses indicate that the ABC transporter belongs to a multidrug resistance protein (MRP) family. CpMRP1 binds bacterial metabolites, notably deoxycholic acid (DCA) that inhibits C. parvum growth. CpMRP1 is exported from small granules to the parasite-host interface, potentially mediating the export of xenobiotics. Loss of CpMRP1 reduces infectivity and DCA resistance in mice, and CpMRP1 polymorphisms across isolates determine susceptibility to DCA. These results define CpMRP1 as a determinant of C. parvum sensitivity to microbiome-mediated inhibition, thereby influencing infectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


