丝氨酸蛋白酶抑制剂抑制铁下垂可减轻急性呼吸窘迫综合征
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
急性呼吸窘迫综合征(ARDS)以高死亡率为特征,涉及多个分子程序,特别是铁中毒-一种由铁过载和脂质过氧化驱动的免疫原性细胞死亡形式。乌司他丁(Ulinastatin, UTI)是一种丝氨酸蛋白酶抑制剂,对ARDS有临床疗效,但其作用机制尚不清楚。我们的目标是在这一过程中发现潜在的分子靶点,以促进ARDS治疗的临床转化。方法我们对lps诱导的ARDS小鼠肺组织进行RNA测序(RNA-seq),发现尿路感染的ARDS小鼠肺组织中铁凋亡相关通路显著富集,提示尿路感染通过抑制铁凋亡来减轻ARDS的假设。采用lps诱导的小鼠ARDS,我们通过组织病理学、qRT-PCR、RNA测序和分子分析来评估UTI的治疗效果。评估铁凋亡生物标志物(铁、MDA、GSH)、关键蛋白(GPX4、KEAP1、NRF2)和炎症因子。体外应用HUVEC和MLE-12研究UTI通过铁下垂发挥作用的分子机制。分子对接探索UTI-KEAP1/NRF2相互作用。结果ti能显著减轻lps刺激小鼠的肺损伤,降低炎症因子(IL-1β、IL-6、TNF-α),恢复肝肾功能。转录组学显示铁下垂是UTI抑制的顶部富集途径。在机制上,在HUVEC和MLE-12细胞中,UTI减弱了lps诱导的不稳定铁、MDA和脂质ROS水平的增加。此外,UTI抑制KEAP1的表达,同时激活NRF2,其作用与铁下垂抑制剂相当。因此,GPX4表达上调,提示其具有潜在的抗铁衰机制。结论抑制铁下垂是UTI对ARDS肺保护作用的新机制。UTI可能通过直接结合KEAP1调控KEAP1- nrf2相互作用,为其作用机制提供了新的分子水平解释。本文章由计算机程序翻译,如有差异,请以英文原文为准。
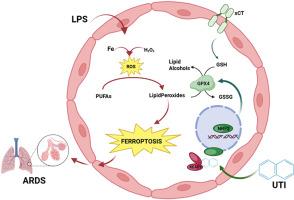
Inhibition of ferroptosis by serine protease inhibitor attenuates acute respiratory distress syndrome
Background
Acute respiratory distress syndrome (ARDS), characterized by high mortality, involves multiple molecular programs, notably ferroptosis—a form of immunogenic cell death driven by iron overload and lipid peroxidation. Ulinastatin (UTI), a serine protease inhibitor, shows clinical efficacy in ARDS, but its underlying mechanism remains unclear. We aim to identify potential molecular targets in this process to promote clinical translation in ARDS treatment.
Methods
We performed RNA sequencing (RNA-seq) on lung tissues from LPS-induced ARDS mice revealed significant enrichment of ferroptosis-related pathways in UTI-treated ARDS mice, prompting the hypothesis that UTI mitigates ARDS by suppressing ferroptosis. Using LPS-induced murine ARDS, we assessed UTI's therapeutic effects via histopathology, qRT-PCR, RNA sequencing, and molecular assays. Ferroptosis biomarkers (iron, MDA, GSH), key proteins (GPX4, KEAP1, NRF2), and inflammatory cytokines were evaluated. In vitro, HUVEC and MLE-12 were used to investigate the molecular mechanisms by which UTI's functions via ferroptosis. Molecular docking explored UTI-KEAP1/NRF2 interactions.
Results
UTI significantly attenuated lung injury, reduced inflammatory cytokines (IL-1β, IL-6, TNF-α), and restored hepatic/renal function in LPS-challenged mice. Transcriptomics revealed ferroptosis as a top enriched pathway suppressed by UTI. Mechanistically, in both HUVEC and MLE-12 cells, UTI attenuated LPS-induced increases in labile iron, MDA, and lipid ROS levels. Additionally, UTI suppressed KEAP1 expression while activating NRF2, an effect comparable to that of ferroptosis inhibitors. Consequently, GPX4 expression was upregulated, suggesting a potential anti-ferroptotic mechanism.
Conclusion
Inhibition of ferroptosis is a novel mechanism underpinning UTI's lung-protective effect against ARDS. UTI potentially regulates the KEAP1-NRF2 interaction through direct binding to KEAP1, offering a new molecular-level explanation for its mechanism of action.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


