抗菌、抗真菌和抗原虫候选药物2-(2-硝基乙烯基)呋喃(G-0)在β-环糊精衍生物固体包合物中的光降解:核磁共振表征和硅毒性筛选
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
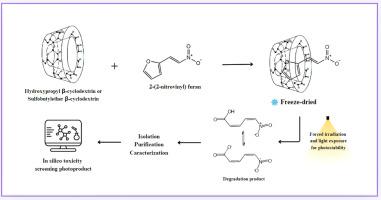
本研究旨在评价候选药物2-(2-硝基)呋喃(G-0)与羟丙基β-环糊精(HP-β- cd)或磺基丁醚β-环糊精(SBE-β- cd)之间包合物(IC)的形成对药物光稳定性的影响。冷冻干燥的环糊精包合物(ic)及其相应的物理混合物根据ICH指南进行标准化的强制照射和光照条件。动力学分析表明光降解途径可能发生改变。与物理混合物相比,ic的HPLC谱图显示出明显的杂质模式,而两种ic的谱图相似。主要光降解产物PD1停留时间为5.4 min,最大吸收波长(λmax)为330 nm。采用高效液相色谱-二极管阵列检测(HPLC-DAD)分离PD1,并通过核磁共振(NMR)光谱进一步表征。结合1D (1H NMR, 13C {1H})和2D (COSY, HSQC和HMBC) NMR测量,发现存在两种平衡物质,确定为(2Z,4Z)-5-硝基戊二烯酸(NPDA)及其相应的盐。根据ICH M7指南,使用QSAR和基于专家规则的毒性筛选方法对NPDA的危害分类进行了评估,提供了对其潜在风险概况的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Photodegradation of the antibacterial, antifungal and antiprotozoal drug candidate 2-(2-nitrovinyl) furan (G-0) in solid inclusion complexes with β-cyclodextrin derivatives: Characterization by NMR spectroscopy and in silico toxicity screening
This work aimed to evaluate the effect of inclusion complex (IC) formation between the drug candidate 2-(2-nitrovinyl) furan (G-0) and hydroxypropyl β-cyclodextrin (HP-β-CD) or sulfobutylether β-cyclodextrin (SBE-β-CD) on the drug’s photostability under light exposure. Freeze-dried cyclodextrin inclusion complexes (ICs) and their corresponding physical mixtures were subjected to standardized forced irradiation and light exposure conditions according to ICH guidelines. Kinetic analysis suggested potential alterations in photodegradation pathways. The HPLC chromatographic profile of the ICs exhibited a distinct impurity pattern compared to the physical mixtures, while the profiles of the two ICs were similar. The main photodegradation product (PD1) was identified with a retention time of 5.4 min and a maximum absorption wavelength (λmax) of 330 nm. PD1 was isolated using High-Performance Liquid Chromatography with diode array detection (HPLC-DAD) and further characterized through Nuclear Magnetic Resonance (NMR) spectroscopy. Combining 1D (1H NMR, 13C {1H}) and 2D (COSY, HSQC and HMBC) NMR measurements revealed the presence of two substances in equilibrium, identified as (2Z,4Z)-5-nitropenta-2,4-dienoic acid (NPDA) and its corresponding salt. The hazard classification of NPDA was assessed following ICH M7 guidelines using QSAR and expert rule-based toxicity screening methods, providing insights into its potential risk profile.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


