间歇式和柱式低成本生物吸附剂对酸性矿井水中pH值的中和和重金属的去除研究
IF 4.9
Q2 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
研究了生牡蛎壳(RO)、牡蛎壳生物炭(OB)和银杏叶生物炭(LB)作为低成本的生物吸附剂对酸性矿井水中重金属的去除和pH的中和。对五种吸附剂进行了批量测试。结果表明,OB、RO和LB对Cd2+(57 ~ 98%)、Cu2+(93 ~ 99%)和Fe2+(96 ~ 99%)的去除率较低,对Mn2+(7 ~ 57%)和Zn2+(18 ~ 97%)的去除率较低。由于其碱性缓冲能力,RO和OB也将溶液pH提高到~ 6.3。在串联柱上进一步检测LB、OB和RO。RO和OB分别在主柱中检测hrt为31.1、93.5和185 min。在93.5 min HRT下测试了LB、OB和RO的混合色谱柱,以提高去除效率。增加HRT可以改善金属去除和突破时间。RO和OB在185 min HRT下从主柱中去除~ 71% Cd2+, ~ 93% Cu2+, ~ 6% Mn2+, ~ 52% Fe2+和~ 11% Zn2+。二级杂化柱中Cd2+去除率为99.53%,Cu2+去除率为100%,Mn2+去除率为55.20%,Fe2+去除率为100%,Zn2+去除率为74.03%。Cu2+ > Fe2+ > Cd2+> Zn2+>; Mn2+为柱型金属去除顺序。在金属吸附过程中,色谱柱的pH值变化明显,表明缓冲过程和酸性金属离子的消除。使用Thomas和Yoon-Nelson方程的柱状模型证实了高吸附能力和延长的突破时间,特别是在混合体系中。SEM-EDS的机理分析表明,在RO和OB表面有Cd2+、Cu2+和Fe2+的沉积和共沉淀,FTIR光谱和XRD谱图证实了碳酸盐、羟基、磷酸基和胺基在金属结合中的作用。LB的多孔结构和官能团通过络合和扩散驱动吸附增强了Mn2+和Zn2+的去除。这些发现支持了牡蛎壳基复合材料作为可持续生物吸附剂修复金属污染、酸性矿山水的潜力,并强调了通过表面功能化和混合处理设计进行优化的未来机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
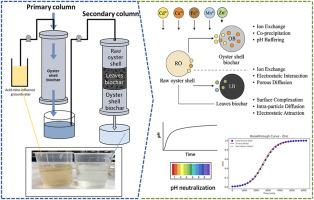
Neutralization of pH and removal of heavy metals from acid mine water by using low-cost biosorbents in batch and column studies
The present research explored raw oyster shell (RO), oyster shell biochar (OB), and ginkgo leaves biochar (LB) as low-cost biosorbents for heavy metal removal and pH neutralization from acid mine water. Five adsorbents were tested in batch mode. The results showed that OB, RO, and LB effectively removed Cd2+ (57–98 %), Cu2+ (93–99 %), and Fe2+ (96–99 %), while Mn2+ (7–57 %) and Zn2+ (18–97 %) exhibited lower removal efficiencies. RO and OB also increased solution pH to ∼6.3 due to their alkaline buffering capacity. LB, OB, and RO were further tested in series columns. RO and OB with 31.1, 93.5, and 185 min HRTs were tested in primary columns. A hybrid column with LB, OB, and RO was tested at 93.5 min HRT to enhance removal efficiency. Increasing HRT improved both metal removal and breakthrough times. RO and OB with 185 min HRT removed ∼71 % Cd2+, ∼93 % Cu2+, ∼6 % Mn2+, ∼52 % Fe2+, and ∼11 % Zn2+ from the primary column. In the secondary hybrid column, 99.53 % Cd2+, 100 % Cu2+, 55.20 % Mn2+, 100 % Fe2+, and 74.03 % Zn2+ were removed. Cu2+ > Fe2+ > Cd2+> Zn2+> Mn2+ was the column mode metal removal order. The columns' pH profiles changed significantly during metal sorption, suggesting buffering processes and acidic metal ion elimination. Column modeling using Thomas and Yoon–Nelson equations confirmed high adsorption capacities and extended breakthrough times, particularly in the hybrid system. Mechanistic analysis via SEM–EDS revealed surface deposition and co-precipitation of Cd2+, Cu2+, and Fe2+ on RO and OB, while FTIR spectra and XRD patterns confirmed the roles of carbonate, hydroxyl, phosphate, and amine groups in metal binding. LB's porous structure and functional groups enhanced Mn2+ and Zn2+ removal through complexation and diffusion-driven sorption. The findings support the potential of oyster shell-based composites as sustainable biosorbents for the remediation of metal-contaminated, acid mine water, and highlight future opportunities for optimization through surface functionalization and hybrid treatment designs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Groundwater for Sustainable Development
Social Sciences-Geography, Planning and Development
CiteScore
11.50
自引率
10.20%
发文量
152
期刊介绍:
Groundwater for Sustainable Development is directed to different stakeholders and professionals, including government and non-governmental organizations, international funding agencies, universities, public water institutions, public health and other public/private sector professionals, and other relevant institutions. It is aimed at professionals, academics and students in the fields of disciplines such as: groundwater and its connection to surface hydrology and environment, soil sciences, engineering, ecology, microbiology, atmospheric sciences, analytical chemistry, hydro-engineering, water technology, environmental ethics, economics, public health, policy, as well as social sciences, legal disciplines, or any other area connected with water issues. The objectives of this journal are to facilitate: • The improvement of effective and sustainable management of water resources across the globe. • The improvement of human access to groundwater resources in adequate quantity and good quality. • The meeting of the increasing demand for drinking and irrigation water needed for food security to contribute to a social and economically sound human development. • The creation of a global inter- and multidisciplinary platform and forum to improve our understanding of groundwater resources and to advocate their effective and sustainable management and protection against contamination. • Interdisciplinary information exchange and to stimulate scientific research in the fields of groundwater related sciences and social and health sciences required to achieve the United Nations Millennium Development Goals for sustainable development.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


