细胞膜工程纳米颗粒:靶向癌症治疗的仿生平台
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-08-23
DOI:10.1016/j.bioadv.2025.214471
引用次数: 0
摘要
尽管在传统和尖端治疗方面取得了进步,但癌症仍然是靶向药物递送最具挑战性的疾病之一。近年来,纳米医学领域取得了快速进展,提供了新的策略,具有通过提高生物利用度、有效性和安全性来改变医疗保健的潜力。值得注意的是,这些纳米治疗药物输送系统的靶向能力已经通过定制纳米级特性和修改表面特征而得到改进,为肿瘤靶向治疗提供了一种替代方法。然而,目前的纳米载体系统仍然存在意想不到的脱靶效应、免疫清除和对几种生物屏障的有限渗透,包括血脑屏障和肿瘤微环境。为了解决这些障碍,仿生方法已经出现,特别是隐藏在生物细胞膜内的纳米颗粒,它模仿自然细胞功能,增强循环半衰期,更有效地与肿瘤微环境相互作用和免疫逃避。除了这些巨大的优势,这些仿生纳米颗粒还面临着持续的挑战,包括制造的可扩展性、潜在的免疫原性和从实验室到临床应用的监管许可。本文综述了近年来基于仿生纳米载体的癌症药物传递系统的研究进展和局限性,重点介绍了膜包覆纳米载体的类型、制备方法、来源、面临的挑战及其在癌症治疗中的应用。总的来说,目前的综述为仿生纳米颗粒的未来研究及其临床应用提供了一个路线图。本文章由计算机程序翻译,如有差异,请以英文原文为准。
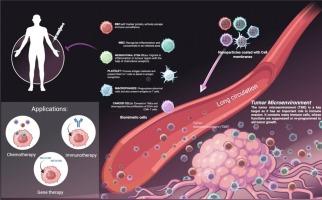
Cell membrane-engineered nanoparticles: A bionic platform for targeted cancer therapy
Cancer remains one of the most challenging diseases for targeted drug delivery despite the advancements in conventional as well as cutting-edge treatments. In recent years, rapid progress has been made in the field of nanomedicine, offering novel strategies, holding the potential to transform healthcare by enhancing bioavailability, efficacy and safety. Notably, the targeting ability of these nanotherapeutic drug delivery systems has been refined by tailoring nanoscale properties and modified surface features, furnishing an alternative approach to tumour-targeted therapy. However, current nanocarrier systems still suffer from unexpected off-target effects, immune clearance, and limited penetration into several biological barriers, including the blood-brain barrier and tumour microenvironment. To address these barriers, the biomimicking approach has come into existence, especially nanoparticles cloaked within the biological cell membranes, which mimic the natural cell function, enabling enhanced circulation half-life, more efficient interaction with the tumour microenvironment and immune evasion. Besides such vast advantages, these biomimicking nanoparticles face ongoing challenges including manufacturing scalability, potential immunogenicity and regulatory clearance for bench to bedside use. This review discusses the recent advancements and limitations of the biomimicking nanocarriers-based drug delivery systems for cancer treatment, especially focused on the cell membrane-coated nanocarriers, covering their types, fabrication methods, sources, and present challenges and their applications in cancer treatments. Overall, the current review offers a roadmap for future research in biomimicking nanoparticles and their clinical implementation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


