替诺福韦与恩替卡韦治疗对慢性乙型肝炎进展的影响:一项全国性队列研究
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
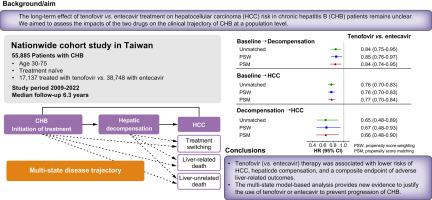
背景和目的在接受替诺福韦和恩替卡韦治疗的慢性乙型肝炎(CHB)患者的肝细胞癌(HCC)风险方面存在相互矛盾的证据。我们在人群水平上评估了这两种药物对慢性乙型肝炎临床发展轨迹的影响。应用多状态模型来评估治疗对疾病进展轨迹的影响,包括CHB(伴/不伴代偿性肝硬化)、肝失代偿、HCC、死亡和切换治疗状态。使用倾向评分匹配/加权和亚组分析来确认研究结果的稳健性。结果在中位6.3年的随访期间,1524例患者发生肝功能失代偿,3591例患者发生HCC, 3436例患者死亡。与恩替卡韦相比,替诺福韦与CHB进展风险较低相关,从基线过渡到肝失代偿和HCC的调整风险比(95% ci)分别为0.84(0.75-0.95)和0.76(0.70-0.83),失代偿引起的HCC风险为0.65(0.48-0.89)。倾向得分匹配/加权分析得出了类似的结果。在未经历失代偿的患者中,通过敏感性分析观察到替诺福韦在多个亚组(包括年龄、性别、糖尿病和肝硬化)中显著降低HCC风险。替诺福韦和恩替卡韦的5年主要肝脏相关不良结局(失代偿、HCC和肝脏相关死亡)风险分别为5.5%和7.5%(校正风险比0.80;95% CI 0.74-0.85)。结论对慢性乙型肝炎严重程度的时间演变进行多状态建模,与恩替卡韦相比,长期替诺福韦治疗与严重肝脏结局的风险较低相关。影响和意义替诺福韦与恩替卡韦预防慢性乙型肝炎(CHB)患者肝细胞癌(HCC)的比较效果仍存在争议。通过一项涉及55,885例慢性乙型肝炎患者的全国性队列研究,我们发现替诺福韦(与恩替卡韦相比)治疗与HCC、肝失代偿和肝脏相关不良结局(肝失代偿、HCC和肝脏相关死亡)的综合终点的风险较低相关,而与年龄、性别、糖尿病、酒精相关疾病和肝硬化状态无关。我们的结果为使用替诺福韦或恩替卡韦预防慢性乙型肝炎严重程度的演变提供了新的证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Impact of tenofovir vs. entecavir treatment on progression of chronic hepatitis B: A nationwide cohort study
Background & Aims
Conflicting evidence exists on hepatocellular carcinoma (HCC) risk in patients with chronic hepatitis B (CHB) receiving tenofovir vs. entecavir. We assessed the impacts of the two drugs on the clinical trajectory of CHB at a population level.
Methods
We conducted a retrospective nationwide cohort study using data from Taiwan’s National Health Insurance Research Database, including 55,885 patients with CHB who were treatment-naïve aged 30–75 years receiving tenofovir (n = 17,137) or entecavir (n = 38,748) monotherapy for ≥3 months between November 2009 and December 2020, and followed until December 2022. Multi-state modeling was applied to evaluate treatment impacts on disease progression trajectory, comprising CHB (with/without compensated cirrhosis), hepatic decompensation, HCC, death, and a switching treatment state. Propensity score matching/weighting and subgroup analyses were used to confirm the robustness of findings.
Results
During a median 6.3 years of follow-up, 1,524 patients developed hepatic decompensation, 3,591 developed HCC, and 3,436 died. Tenofovir compared with entecavir was associated with lower risk of CHB progression, with adjusted-hazard ratios (95% CIs) of 0.84 (0.75–0.95) and 0.76 (0.70–0.83), respectively, for transitions to hepatic decompensation and HCC from baseline, and 0.65 (0.48–0.89) for HCC risk from decompensation. Propensity score matching/weighting analyses yielded similar results. Among patients without experiencing decompensation, a significantly lower HCC risk with tenofovir was observed across multiple subgroups, including age, sex, diabetes, and cirrhosis, and by sensitivity analyses. The 5-year risk of major adverse liver-related outcomes (decompensation, HCC, and liver-related deaths) was 5.5% and 7.5% for tenofovir and entecavir, respectively (adjusted-hazard ratio 0.80; 95% CI 0.74–0.85).
Conclusions
Using multi-state modeling on the temporal evolution of CHB severity, long-term tenofovir treatment compared with entecavir was associated with a lower risk of severe liver outcomes.
Impact and implications
The comparative effectiveness of tenofovir vs. entecavir treatment in preventing hepatocellular carcinoma (HCC) for patients with chronic hepatitis B (CHB) remains controversial. Using a nationwide cohort study involving 55,885 patients to investigate CHB as a multi-state disease over 13 years, we show that tenofovir (vs. entecavir) treatment is associated with lower risks of HCC, hepatic decompensation, and a composite endpoint of adverse liver-related outcomes (hepatic decompensation, HCC, and liver-related deaths), regardless of age, sex, diabetes, alcohol-related disorders, and cirrhosis state. Our results provide new evidence justifying the use of tenofovir or entecavir to prevent the evolution of CHB severity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


