肿瘤细胞微环境响应型生物金属-有机骨架抗癌药物传递
Q3 Materials Science
引用次数: 0
摘要
采用生物相容性l-胱氨酸和锌制备了谷胱甘肽(GSH)响应型金属-有机骨架(MOF),用于靶向递送阿霉素(DOX)。这种策略增强了药物在微环境中的释放,同时最小化了全身毒性。以l -胱氨酸为配体,锌为金属节点,合成了含二硫化物的mof。在合成过程中,采用一锅法包封DOX。在生理和肿瘤模拟条件下评估所得DOX@MOF-Zn(胱氨酸)的氧化还原反应行为。体外实验表明,l -胱氨酸配体中的二硫键在GSH存在下促进MOF降解,触发DOX释放。与生理条件(pH 7.4,无GSH)相比,DOX@MOF-Zn(胱氨酸)在模拟肿瘤微环境(pH 5.4, 20 mM GSH)下的释放明显增强。细胞活力测试表明,DOX@MOF-Zn(胱氨酸)对空白mof的毒性很小,具有很强的dox依赖性抗癌作用。这些发现表明DOX@MOF-Zn(胱氨酸)是一种有效的gsh反应性药物递送系统,用于靶向癌症治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
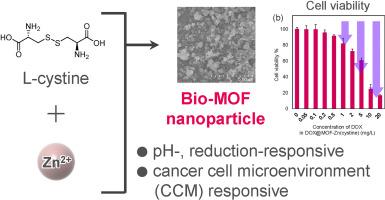
Cancer cell microenvironment-responsive bio-metal-organic frameworks for anticancer drug delivery
A glutathione (GSH)-responsive metal-organic framework (MOF) was developed using biocompatible L-cystine and Zn for targeted doxorubicin (DOX) delivery. This strategy enhances drug release in the microenvironment while minimizing systemic toxicity. Disulfide-containing MOFs were synthesized using L-cystine as the ligand and Zn as the metal node. A one-pot method was developed to encapsulate DOX during synthesis. The redox-responsive behavior of the resulting DOX@MOF-Zn(cystine) was evaluated under physiological and tumor-mimicking conditions. In vitro assays revealed that the disulfide bonds in L-cystine ligand facilitate MOF degradation in the presence of GSH, triggering DOX release. DOX@MOF-Zn(cystine) exhibited significantly enhanced release under conditions mimicking the tumor microenvironment (pH 5.4, 20 mM GSH) compared to physiological conditions (pH 7.4, no GSH). Cell viability assays demonstrated minimal toxicity for blank MOFs and strong DOX-dependent anticancer effects from DOX@MOF-Zn(cystine). These findings suggest DOX@MOF-Zn(cystine) is an effective GSH-responsive drug delivery system for targeted cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JCIS open
Physical and Theoretical Chemistry, Colloid and Surface Chemistry, Surfaces, Coatings and Films
CiteScore
4.10
自引率
0.00%
发文量
0
审稿时长
36 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


