头颈部鳞状细胞癌的免疫治疗和抗衰老药物:2期试验结果
IF 50
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
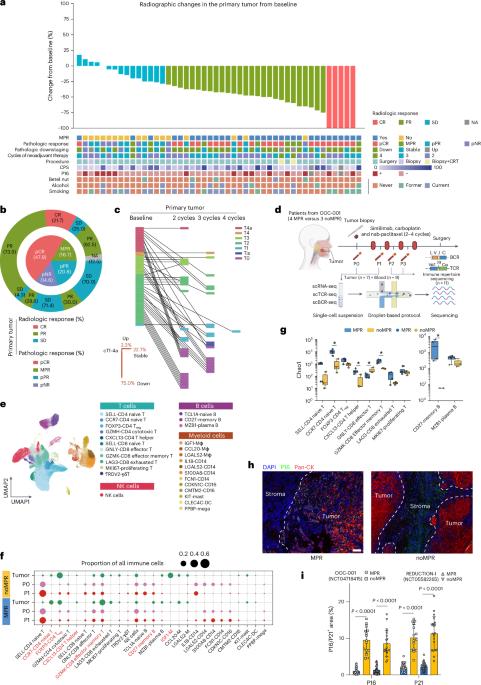
癌症免疫治疗的最新进展改善了患者的预后,但对免疫治疗的反应仍然温和。免疫衰老已被证明有助于各种疾病的发生和进展;然而,其在实体瘤中的具体作用尚未完全确定。在这里,我们进行了一项涉及51例接受新辅助化疗免疫治疗的癌症患者的2期临床试验,并对肿瘤和血液样本应用单细胞RNA以及TCR和BCR测序来阐明免疫细胞的扰动。我们的研究结果表明,不良反应与CCR7+ CD4+幼稚T细胞和CD27+记忆B细胞水平降低以及T细胞和B细胞亚群中免疫衰老相关基因的高表达有关。使用自然衰老小鼠和ercc1缺陷小鼠(早衰),我们发现抗衰老剂通过减轻免疫衰老来增强多发性实体瘤的免疫治疗效果。值得注意的是,我们启动了一项2期临床试验(COIS-01),研究抗衰老药物与抗pd -1治疗的联合应用。结果显示,联合治疗的主要病理反应率为33.3%(95%可信区间16.6-54.7%),3-4级不良事件发生率较低(4.2%)。这些发现强调了免疫衰老特征在影响免疫治疗效果中的关键作用,并表明抗pd -1联合抗衰老药物具有良好的疗效和安全性。ClinicalTrials.gov标识符:OOC-001(NCT04718415)和COIS-01(NCT05724329)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Immunotherapy and senolytics in head and neck squamous cell carcinoma: phase 2 trial results
Recent advancements in cancer immunotherapy have improved patient outcomes, yet responses to immunotherapy remain moderate. Immunosenescence has been shown to contribute to the development and progression of various diseases; however, its specific role in solid tumors has not been fully delineated. Here we conducted a phase 2 clinical trial involving 51 patients with cancer undergoing neoadjuvant chemoimmunotherapy and applied single-cell RNA as well as TCR and BCR sequencing on tumor and blood samples to elucidate the immune cell perturbations. Our findings associate poor response with reduced levels of CCR7+ CD4+ naive T cells and CD27+ memory B cells, as well as higher expression of immunosenescence-related genes in T and B cell subsets. Using naturally aged mice and Ercc1-deficient mice (premature aging), we found that senolytics enhance the therapeutic efficacy of immunotherapy in multiple solid tumors by mitigating immunosenescence. Notably, we launched a phase 2 clinical trial (COIS-01) investigating the combination of senolytics with anti-PD-1 therapy. The results showed that the combination therapy achieved a 33.3% (95% confidence interval 16.6–54.7%) major pathological response rate with a low incidence of grade 3–4 adverse events (4.2%). These findings underscore the pivotal role of immunosenescence characteristics in influencing the effectiveness of immunotherapy and suggest a promising therapeutic efficacy along with a favorable safety for the combination of senolytics with anti-PD-1 therapy. ClinicalTrials.gov Identifier: OOC-001( NCT04718415 ) and COIS-01( NCT05724329 ). Two phase 2 trials, along with translational analysis of prospective cohorts and experimental analysis, indicate that immunosenescence as a mechanism of resistance to immunotherapy can be overcome with the senolytics dasatinib and quercetin in patients with head and neck squamous cell carcinoma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


