协同相分离Cu-Zn催化剂选择性CO2电化学还原乙醇:界面位置作用的理论见解
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
双金属铜锌电催化剂已成为选择性还原二氧化碳(CO2)为乙醇的有希望的候选人。虽然在单金属Cu和Zn催化剂及其均相合金上对CO2还原反应(CO2RR)进行了广泛的研究,但非均相Cu - Zn界面对CO2还原反应的影响尚不充分。本文采用密度泛函理论(DFT)计算研究了相分离Cu-Zn催化剂对乙醇催化CO2RR的界面效应。界面位点显著调节中间稳定性并增强C-C耦合步骤,特别是CO-CH耦合,这在热力学和动力学上都是有利的(ΔGrxn =−1.28 eV; ΔG‡= 0.27 eV)。在随后的*CHCO形成后,界面位置更倾向于乙醇途径而不是乙烯。微动力学分析进一步表明,CO-CH偶联对乙醇生产起主要作用。这些发现揭示了界面位点在选择性乙醇生产中的协同作用,为Cu-Zn电催化剂的合理设计提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
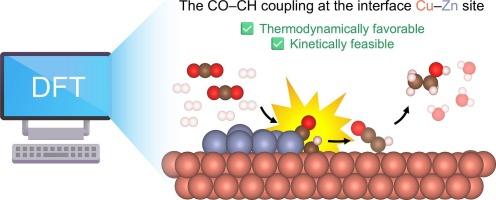
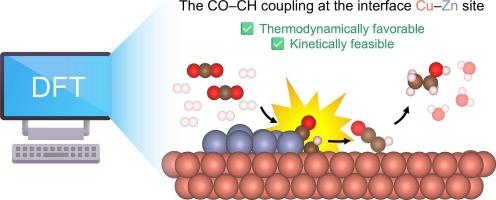
Cooperative phase-separated Cu–Zn catalyst for selective CO2 electrochemical reduction toward ethanol: theoretical insights into the role of the interface sites
Bimetallic Cu–Zn electrocatalysts have emerged as promising candidates for selectively reducing carbon dioxide (CO2) to ethanol. While CO2 reduction reaction (CO2RR) has been studied extensively on monometallic Cu and Zn catalysts and their homogenous alloys, the influence of heterogeneous Cu–Zn interfaces remain underexplored. Herein, we used density functional theory (DFT) calculations to investigate the interface effects of phase-separated Cu–Zn catalysts on CO2RR toward ethanol. Interface sites significantly modulate intermediate stability and enhance the C–C coupling step, particularly CO–CH coupling, which is both thermodynamically and kinetically favorable (ΔGrxn = −1.28 eV; ΔG‡ = 0.27 eV). Interface site favors the ethanol pathway over ethylene after subsequent *CHCO formation. Microkinetic analysis further suggests that CO–CH coupling dominantly contributes to the ethanol production. These findings reveal the cooperative role of interface sites in enabling selective ethanol production, providing insights into the rational design of Cu–Zn electrocatalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


