电化学CO2还原与甘油氧化相结合:今天是瓶颈,明天是机遇
IF 35.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
阻碍二氧化碳电解工业应用的最大障碍之一是电池电压大(通常超过3v),这主要源于阳极发生的析氧反应(OER)的高氧化还原电位和过电位。可以通过替代OER的方法来缓解这一问题,例如小有机分子的电化学氧化。虽然配对CO2还原反应(CO2RR)/小有机分子氧化的例子数量正在迅速增加,但它们的生存能力仅在实验室规模上进行了测试,主要是在批式细胞中。从这个角度出发,以甘油氧化反应(GOR)为例,总结了GOR产品定量、粗甘油作为原料的使用以及GOR与CO2RR的整合等方面面临的主要挑战。最后,概述了大规模实施配对CO2RR/GOR电解的最重要设想的未来步骤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
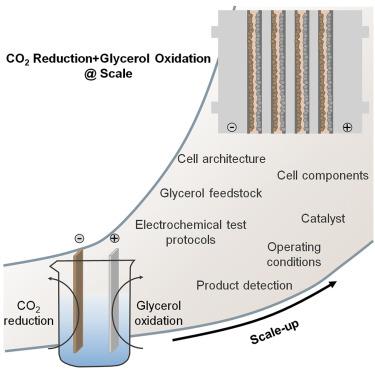
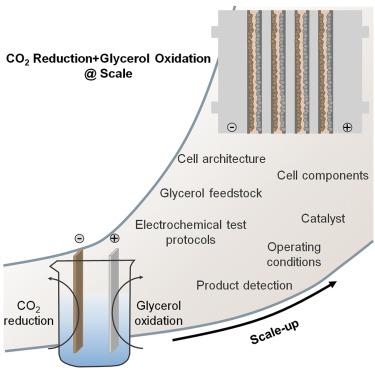
Pairing electrochemical CO2 reduction with glycerol oxidation: Bottlenecks today, opportunities tomorrow
One of the greatest obstacles hampering the industrial application of CO2 electrolysis is the large cell voltage (often over 3 V), which is mainly rooted in the high redox potential and overpotential of the oxygen evolution reaction (OER) occurring at the anode. It is possible to mitigate this issue by replacing the OER with alternative processes, such as the electrochemical oxidation of small organic molecules. Although the number of examples of paired CO2 reduction reaction (CO2RR)/small organic molecule oxidation is rapidly increasing, their viability has only been tested at the laboratory scale, mainly in batch cells. In this perspective, taking the glycerol oxidation reaction (GOR) as an example, the main challenges concerning the quantification of GOR products, the use of crude glycerol as a feedstock, and the integration of GOR with CO2RR are summarized. Finally, the most important envisioned future steps for the implementation of paired CO2RR/GOR electrolysis at scale are outlined.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Joule
Energy-General Energy
CiteScore
53.10
自引率
2.00%
发文量
198
期刊介绍:
Joule is a sister journal to Cell that focuses on research, analysis, and ideas related to sustainable energy. It aims to address the global challenge of the need for more sustainable energy solutions. Joule is a forward-looking journal that bridges disciplines and scales of energy research. It connects researchers and analysts working on scientific, technical, economic, policy, and social challenges related to sustainable energy. The journal covers a wide range of energy research, from fundamental laboratory studies on energy conversion and storage to global-level analysis. Joule aims to highlight and amplify the implications, challenges, and opportunities of novel energy research for different groups in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


