内质网中多伴侣凝聚物的发现
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
如何组织多个伴侣来协调他们的活动尚不清楚。我们观察到伴侣PDIA6在内质网中形成相分离凝聚体,其中招募了几个额外的伴侣。这些多伴侣蛋白凝聚体构成了一个专用的内质网亚室,促进蛋白质的生物生成,防止蛋白质错误折叠和聚集。本文章由计算机程序翻译,如有差异,请以英文原文为准。

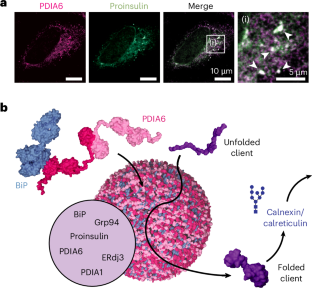
Discovery of a multi-chaperone condensate in the endoplasmic reticulum
How multiple chaperones are organized to co-ordinate their activities has been unclear. We observed that the chaperone PDIA6 forms phase-separated condensates in the endoplasmic reticulum to which several additional chaperones are recruited. These multi-chaperone condensates constitute a dedicated endoplasmic reticulum sub-compartment that facilitates protein biogenesis and prevents protein misfolding and aggregation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


