靶向ecm产生细胞的CAR-T治疗可缓解慢性肾脏疾病的纤维化
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
肾纤维化是慢性肾脏疾病(CKD)的标志,也是一个潜在的治疗靶点。然而,由于瘢痕形成细胞的复杂起源、功能异质性和调控,针对肾纤维化的临床干预和治疗仍然是概念和实践上的挑战。在这里,我们将成纤维细胞、周细胞和肌成纤维细胞定义为肾脏中主要的细胞外基质(ECM)产生细胞,强调它们对肾脏纤维化的主要贡献。然后,我们确定血小板衍生生长因子受体β (PDGFRβ)作为抗纤维化嵌合抗原受体(CAR)-T治疗CKD的潜在靶向表面抗原。在多种小鼠CKD模型中,过养性转移和cd5 -脂质纳米颗粒(LNP)介导的PDGFRβ CAR-T细胞的体内生成均可显著改善纤维化相关病理,包括肾脏、心肌间质和血管周围纤维化,且无明显毒性,为CKD小鼠多器官纤维化及其心血管并发症提供了一种综合治疗策略。抗纤维化作用也在人类肾脏类器官CKD中得到证实,进一步有力地支持了治疗CKD患者的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
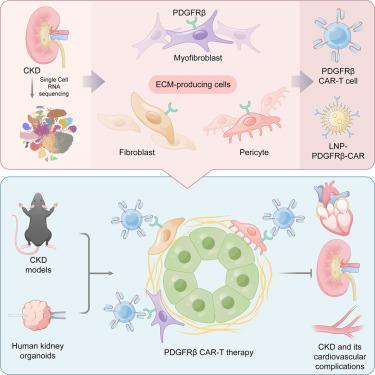
Targeting ECM-producing cells with CAR-T therapy alleviates fibrosis in chronic kidney disease
Kidney fibrosis is a hallmark of chronic kidney disease (CKD) and a potential therapeutic target. However, clinical interventions and therapies targeting kidney fibrosis remain conceptual and practical challenges due to the complex origin, functional heterogeneity, and regulation of scar-forming cells. Here, we define fibroblasts, pericytes, and myofibroblasts as the major extracellular matrix (ECM)-producing cells in the kidney, highlighting their primary contribution to kidney fibrosis. We then identify platelet-derived growth factor receptor β (PDGFRβ) as a potential targeting surface antigen for anti-fibrotic chimeric antigen receptor (CAR)-T against CKD. In multiple mouse CKD models, both adoptive transfer and CD5-lipid nanoparticle (LNP)-mediated in vivo generation of PDGFRβ CAR-T cells significantly ameliorate fibrosis-associated pathologies, including kidney, myocardial interstitial, and perivascular fibrosis without notable toxicity, evoking an integrated therapeutic strategy for multi-organ fibrosis in mice with CKD and its cardiovascular complications. The anti-fibrotic effects are also demonstrated in the human kidney organoid CKD, further strongly supporting the therapeutic potential for the treatment of patients with CKD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


