基于ipsc的异基因细胞治疗帕金森病临床试验中免疫反应的控制
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
由于中枢神经系统(CNS)是一种免疫特权器官,与器官移植相比,使用诱导多能干细胞(iPSCs)进行细胞治疗需要不同的免疫抑制策略。我们最近进行了首个使用同种异体iPSCs治疗帕金森病的人体临床试验(jRCT编号:jRCT2090220384)。所有患者均通过他克莫司单独免疫抑制移植具有纯合子人白细胞抗原(HLA)单倍型的多能干细胞分化的多巴胺能神经祖细胞(iPSC-DANs)。无论HLA相容性如何,本研究均未观察到有临床意义的免疫反应。然而,使用ipsc衍生的树突状细胞作为刺激物的高度敏感的混合淋巴细胞反应表明,来自hla错配受体的淋巴细胞被激活。这一发现表明,HLA在iPSC-DANs中的低表达有助于成功植入免疫特权中枢神经系统。这些结果表明,干细胞移植到中枢神经系统可能只需要适度的免疫抑制治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
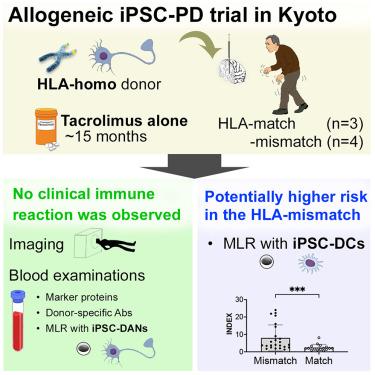
Control of immune response in an iPSC-based allogeneic cell therapy clinical trial for Parkinson’s disease
Because the central nervous system (CNS) is an immune-privileged organ, it requires different immunosuppression strategies for cell therapies using induced pluripotent stem cells (iPSCs) compared with ones for organ transplantations. We recently conducted the first in-human clinical trial of a cell therapy for Parkinson’s disease using allogeneic iPSCs (jRCT number: jRCT2090220384). All patients were transplanted with dopaminergic neural progenitors differentiated from iPSCs (iPSC-DANs), which had homozygous human leukocyte antigen (HLA) haplotypes, through immunosuppression with tacrolimus alone. No clinically significant immune reaction was observed in this study, regardless of HLA compatibility. However, a highly sensitive mixed lymphocyte reaction using iPSC-derived dendritic cells as a stimulator demonstrated the activation of lymphocytes from HLA-mismatch-grafted recipients. This finding suggests that the low expression of HLA in iPSC-DANs contributes to successful engraftment in the immune-privileged CNS. These results indicate that only moderate immunosuppressive treatment may be required for stem cell transplantation to the CNS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


