StBPA1可减弱马铃薯表面受体的激活并精细调节对细菌和卵菌病原体的免疫反应
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
植物利用质膜定位模式识别受体(PRRs)来感知和响应微生物感染。下游调控成分已被广泛研究,但确保对不同病原体作出适当免疫反应的机制仍然是谜。我们报道了一个名为StBPA1 (ACD11的BINDING PARTNER)的核心调控成分是一个控制抗菌和抗卵霉菌免疫的分子开关。stbpa1基因敲除显示出矮小的生长,增强的模式触发免疫(PTI),以及对马铃薯细菌性枯萎病和晚疫病的广谱抗性。StBPA1负性调节鞭毛蛋白感应2 (StFLS2)-BRI1-ASSOCIATED KINASE 1 (StBAK1)/ BIR1-1抑制因子(StSOBIR1)-StBAK1免疫复合物的形成,抑制StFLS2激酶活性,阻止构成性免疫应答。反过来,StBAK1特异性磷酸化StBPA1的Thr193/Ser195。这种修饰被flg22/INF1感知增强,并削弱StBPA1的负调控作用,从而确保适当的免疫信号。这些发现确定了StBPA1- prr复合物调控模块,并强调了StBPA1的抑制作用是确保针对不同病原体的有效且严格调控的免疫反应的关键机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
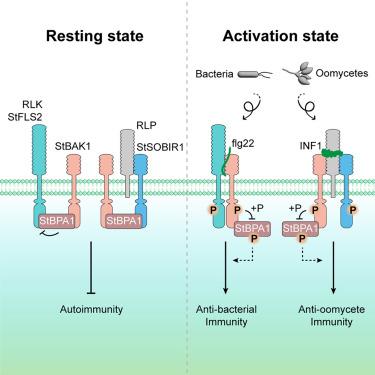
StBPA1 attenuates surface receptor activation and finely regulates immune responses against bacterial and oomycete pathogens in potato
Plants utilize plasma-membrane-localized pattern recognition receptors (PRRs) to sense and respond to microbial infections. The downstream regulatory components have been studied extensively, but the mechanisms ensuring appropriate immune responses to diverse pathogens remain enigmatic. We report that a core regulatory component named StBPA1 (BINDING PARTNER OF ACD11) is a molecular switch that controls both anti-bacterial and anti-oomycete immunity. StBPA1- knockout displays dwarfed growth, enhanced pattern-triggered immunity (PTI), and broad-spectrum resistance to potato bacterial wilt and late blight diseases. StBPA1 negatively regulates the FLAGELLIN SENSING 2 (StFLS2)-BRI1-ASSOCIATED KINASE 1 (StBAK1)/SUPPRESSOR OF BIR1-1 (StSOBIR1)-StBAK1 immune complex formation and inhibits StFLS2 kinase activity to prevent constitutive immune responses. In turn, StBAK1 specifically phosphorylates StBPA1 at Thr193 /Ser195 . This modification is enhanced by flg22/INF1 perception and impairs the negative regulatory role of StBPA1, thereby ensuring proper immune signaling. These findings identify an StBPA1-PRR complex regulatory module and highlight inhibitions by StBPA1 as key mechanisms to ensure efficient yet strictly regulated immune responses against different pathogens.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


