弓形虫感染和慢性IL-1升高驱动海马DNA双链断裂信号,导致认知缺陷
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
由感染引起的慢性炎症,其特征是白细胞介素-1 (IL-1)等细胞因子水平升高,但对于IL-1如何通过表观遗传过程导致认知障碍知之甚少。本研究表明,长期感染弓形虫的小鼠表现出空间记忆受损,这依赖于神经元IL-1信号传导,并通过长期暴露于IL-1β来模拟。弓形虫感染和慢性IL-1β均驱动H2A。海马神经元中x依赖的DNA双链断裂信号和神经元H2A的失效。依赖于x的信号可以阻止由任何一种暴露引起的记忆损伤。我们的研究结果强调了细胞因子诱导的双链断裂依赖信号在空间记忆缺陷中的重要作用,这可能与多种脑部疾病有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。

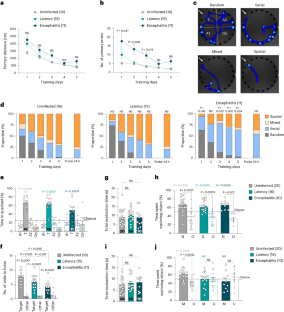
Toxoplasma gondii infection and chronic IL-1 elevation drive hippocampal DNA double-strand break signaling, leading to cognitive deficits
Chronic inflammation, resulting from infections, is characterized by increased levels of cytokines including interleukin-1 (IL-1), but little is known about how IL-1 contributes to cognitive impairment, potentially via epigenetic processes. Here we demonstrate that mice chronically infected with the parasite Toxoplasma gondii exhibit impaired spatial memory, which is dependent on neuronal IL-1 signaling and mimicked by chronic exposure to IL-1β. Both T. gondii infection and chronic IL-1β drive H2A.X-dependent DNA double-strand break signaling in hippocampal neurons and invalidating neuronal H2A.X-dependent signaling blocks memory impairments caused by either exposure. Our results highlight the instrumental role of cytokine-induced double-strand-break-dependent signaling in spatial memory defects, which may be relevant to multiple brain diseases. Chronic brain infection and IL-1 exposure impair spatial memory by triggering DNA double-strand break signaling in hippocampal neurons. Blocking this pathway prevents memory deficits, suggesting new therapeutic prospects for various brain diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


