无细胞合成的人β1-肾上腺素能受体与Gs复合物的低温电镜结构
IF 4.3
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
β1-肾上腺素能受体(β1AR)的第三胞内环(ICL3)在调节G蛋白偶联中起关键作用,但由于与G蛋白或G蛋白模拟纳米体复合物的β1AR的所有可用结构中ICL3都被截断,其结构基础尚不清楚。为了解决这一问题,我们将全长人β1AR无细胞共翻译插入到纳米圆盘中,并测定了其与Gs复合物的冷冻电镜(cro - em)结构。在这种结构中,ICL3延伸了跨膜螺旋5,从而增强了与Gαs的相互作用,并使参与的G蛋白轻微旋转。这种重新定位使得Gαs、ICL2和螺旋8之间产生了新的极性相互作用,而ICL1和螺旋8与Gβ形成了额外的接触。这些突变分析支持的结构见解表明,ICL3通过促进受体与异源三聚体G蛋白之间更广泛的相互作用,增强了G蛋白的激活和下游cAMP信号传导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
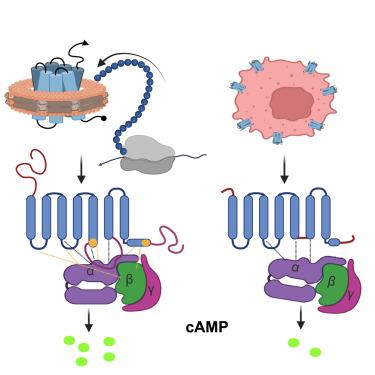
Cryo-EM structure of a cell-free synthesized full-length human β1-adrenergic receptor in complex with Gs
The third intracellular loop (ICL3) of the β1-adrenergic receptor (β1AR) plays a critical role in regulating G protein coupling, yet the structural basis has remained unclear due to truncations of ICL3 in all available structures of the β1AR in complex with Gs or a G protein mimetic nanobody. To address this, we used cell-free cotranslational insertion of full-length human β1AR into nanodiscs and determined its cryo-electron microscopy (cryo-EM) structure in complex with Gs. In this structure, ICL3 extends transmembrane helix 5, resulting in enhanced interactions with Gαs and in a slight rotation of the engaged G protein. This repositioning enables new polar interactions between Gαs, ICL2 and helix 8, while ICL1 and helix 8 form additional contacts with Gβ. These structural insights, supported by mutational analysis, demonstrate that ICL3 enhances G protein activation and downstream cAMP signaling by promoting more extensive interactions between the receptor and the heterotrimeric G protein.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


