蛋白伴侣网络多靶点肽调节剂的设计
IF 4.3
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
必需伴侣蛋白热休克蛋白70 (Hsp70)和热休克蛋白90 (Hsp90)共同参与癌蛋白折叠。这些伴侣的双重抑制在临床前研究中显示出协同作用,但仍然具有挑战性。使用计算方法,我们设计了模拟Hsp70和Hsp90的客户蛋白激酶CDK4的预测展开区域的肽。肽Cdk4-2被证明可以同时结合Hsp70、Hsp90和共伴侣Cdc37。Cdk4-2具有细胞膜渗透性,可抑制cdk4介导的视网膜母细胞瘤磷酸化,并诱导肾癌细胞凋亡。结构-功能研究确定了Hsp70结合的最小药效团和肽亲和力的关键相互作用。这些发现证明了合理设计伴侣网络多目标调制器的可行性。Cdk4-2是治疗发展的一个有希望的先导,扩大了癌症相关多蛋白机制的调节剂的分子空间。虽然我们关注的是伴侣蛋白,但我们的策略背后的想法是通用的,可以立即转移到其他多蛋白靶点和网络上。本文章由计算机程序翻译,如有差异,请以英文原文为准。
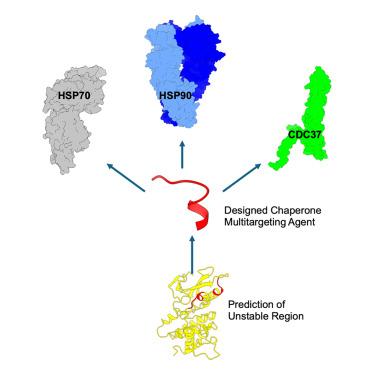
Design of multi-target peptide modulators for protein chaperone networks
Essential chaperones heat shock protein 70 (Hsp70) and heat shock protein 90 (Hsp90) collaborate in oncoprotein folding. Dual inhibition of these chaperones has shown synergy in preclinical studies but remains challenging to achieve. Using a computational approach, we designed peptides mimicking the predicted unfolding regions of Kinase CDK4, a client protein of both Hsp70 and Hsp90. Peptide Cdk4-2 is shown to simultaneously bind Hsp70, Hsp90, and co-chaperone Cdc37. Cdk4-2 is membrane permeable, inhibits CDK4-mediated retinoblastoma phosphorylation, and induces apoptosis in renal carcinoma cells. Structure-function studies identified a minimal pharmacophore for Hsp70 binding and critical interactions for peptide affinity. These findings demonstrate the feasibility of rationally designing multi-target modulators of chaperone networks. Cdk4-2 is a promising lead for therapeutic development, expanding the molecular space of modulators of cancer-associated multiprotein machineries. While focused on chaperones, the idea behind our strategy is general and immediately transferable to other multiprotein targets and networks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


