分泌刺激通过结构重塑明显调节胰岛素分泌颗粒成熟
IF 4.3
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
胰岛素分泌颗粒(ISG)成熟是胰岛素分泌和葡萄糖稳态的关键方面。这种成熟的调控仍然知之甚少,特别是分泌刺激如何影响ISG成熟和亚细胞定位。在本研究中,我们使用软x射线断层扫描(SXT)定量绘制了在各种分泌刺激作用下单个INS-1E和小鼠胰腺β细胞中ISG的形态、密度和位置。我们发现葡萄糖激酶(GK)、胃抑制多肽受体(GIPR)、胰高血糖素样肽-1受体(GLP-1R)和G蛋白偶联受体40 (GPR40)的激活促进了ISG的成熟。每种刺激通过改变大小和密度,根据激活的特定信号级联,诱导isg独特的结构重塑。这些不同的ISG亚群在细胞中动员和重新分布,改变了整个细胞的结构组织。我们的研究结果为当前糖尿病和肥胖治疗如何影响ISG成熟提供了见解,并可能为未来专门针对成熟的治疗提供信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
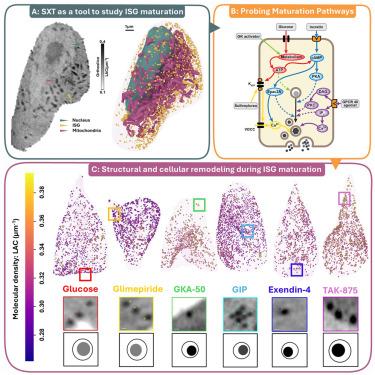
Secretory stimuli distinctly regulate insulin secretory granule maturation through structural remodeling
Insulin secretory granule (ISG) maturation is a crucial aspect of insulin secretion and glucose homeostasis. The regulation of this maturation remains poorly understood, especially how secretory stimuli affect ISG maturity and subcellular localization. In this study, we used soft X-ray tomography (SXT) to quantitatively map ISG morphology, density, and location in single INS-1E and mouse pancreatic β cells under the effect of various secretory stimuli. We found that the activation of glucokinase (GK), gastric inhibitory polypeptide receptor (GIPR), glucagon-like peptide-1 receptor (GLP-1R), and G protein-coupled receptor 40 (GPR40) promotes ISG maturation. Each stimulus induces unique structural remodeling in ISGs, by altering size and density, depending on the specific signaling cascades activated. These distinct ISG subpopulations mobilize and redistribute in the cell, altering the overall cellular structural organization. Our results provide insight into how current diabetes and obesity therapies impact ISG maturation and may inform the development of future treatments that target maturation specifically.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


