被公共卫生监测忽视的哺乳动物中病原体和抗生素耐药基因的广泛跨物种传播
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
非传统养殖和野生哺乳动物在病原体监测中往往被忽视。通过对973例无症状哺乳动物的粪便和组织样本进行宏基因组和亚转录组测序,我们鉴定出128种病毒(30种新病毒),包括一个新的冠状病毒属,10,255种细菌(超过7,000种未描述),201种真菌和7种寄生虫。养殖哺乳动物和野生哺乳动物共有13.3%的病毒种类,其中包括亚洲黑熊的犬冠状病毒和家兔的Getah病毒,而野生豹猫中发现了H5N1禽流感病毒的2.3.4.4b分支。我们确定了养殖和野生哺乳动物之间潜在的细菌病原体传播,以及与人类中发现的细菌具有高度遗传相似性的菌株。我们观察到哺乳动物微生物组中157个临床优先抗生素耐药基因(ARGs)与人类微生物组中ARGs的一致性大于99%,通常与移动遗传元件共存。总的来说,这项工作强调了人与动物界面的跨物种风险。本文章由计算机程序翻译,如有差异,请以英文原文为准。
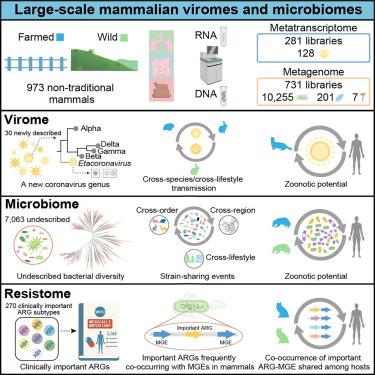
Extensive cross-species transmission of pathogens and antibiotic resistance genes in mammals neglected by public health surveillance
Non-traditional farmed and wild mammals are often neglected in pathogen surveillance. Through metagenomic and metatranscriptomic sequencing of fecal and tissue samples from 973 asymptomatic mammals, we identified 128 viruses (30 novel), including a new coronavirus genus, 10,255 bacterial species (over 7,000 undescribed), 201 fungi, and 7 parasites. Farmed and wild mammals shared 13.3% of virus species, including canine coronavirus in Asiatic black bears and Getah virus in rabbits, while the 2.3.4.4b clade of H5N1 avian influenza virus was found in a wild leopard cat. We identified potential bacterial pathogen transmission between farmed and wild mammals and bacterial strains with high genetic similarity to those found in humans. We observed 157 clinically prioritized antibiotic resistance genes (ARGs) in mammalian microbiomes with greater than 99% identity to ARGs from human microbiomes, often co-occurring with mobile genetic elements. Overall, this work highlights cross-species risks at the human-animal interface.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


