反义寡核苷酸治疗对c9orf72相关ALS的分子影响
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
c9orf72相关肌萎缩性侧索硬化症(c9ALS)是由内含子G4C2重复扩增引起的,该扩增导致毒性RNA转录物和二肽重复蛋白(DPRs)。一项使用反义寡核苷酸(ASO) BIIB078靶向这些转录本的临床试验因未能提供临床益处而终止。在这里,我们确定了靶点参与中枢神经系统(CNS)的程度,并阐明了治疗后脑脊液(CSF)生物标志物的药效学。biib078治疗病例的脑脊液显示dpr降低,炎症生物标志物持续增加,包括C-C基序趋化因子配体26 (CCL26)。BIIB078广泛分布于死后中枢神经系统组织;然而,DPRs和磷酸化的TDP-43仍然丰富。治疗没有改变c9ALS脊髓的蛋白质组学特征,尽管观察到RNase T2丰度明显增加,与BIIB078浓度相关。因此,尽管BIIB078分布广泛,但对关键的中枢神经系统病理没有显著影响,这强调了鉴定反映ASO治疗对疾病相关神经病理变化的药理学生物标志物的必要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
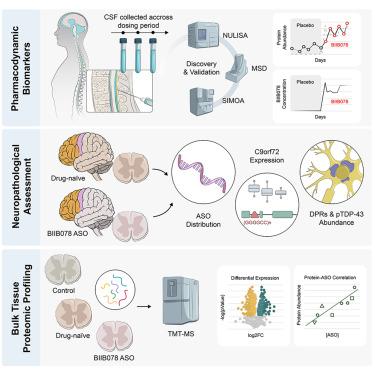
Molecular impact of antisense oligonucleotide therapy in C9orf72-associated ALS
C9orf72-associated amyotrophic lateral sclerosis (c9ALS) is caused by an intronic G4C2 repeat expansion that leads to toxic RNA transcripts and dipeptide repeat proteins (DPRs). A clinical trial using the antisense oligonucleotide (ASO) BIIB078 to target these transcripts was discontinued after failing to provide clinical benefit. Here, we determine the extent of target engagement in the central nervous system (CNS) and elucidate pharmacodynamic cerebrospinal fluid (CSF) biomarkers following treatment. CSF from BIIB078-treated cases showed reduced DPRs and sustained increases in inflammatory biomarkers, including C-C motif chemokine ligand 26 (CCL26). BIIB078 was widely distributed in postmortem CNS tissue; however, DPRs and phosphorylated TDP-43 remained abundant. Proteomic signatures in c9ALS spinal cord were not altered with treatment, although a distinct increase in RNase T2 abundance that correlated with BIIB078 concentration was observed. Thus, despite widespread distribution, BIIB078 did not significantly impact key CNS pathologies, emphasizing the need to identify pharmacodynamic biomarkers that reflect disease-relevant neuropathological changes in response to ASO therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


