线粒体活动调节伤害感受器对兴奋性毒性的恢复能力
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
辣椒素受体TRPV1通过痛觉感受器(疼痛通路的主要感觉神经元)介导对有害化学物质和热刺激的检测。TRPV1的过度激活通过钙进入和兴奋性毒性导致细胞损伤或死亡。我们利用这一现象,通过全基因组CRISPRi筛选对兴奋性毒性进行了系统分析,从而揭示了一个全面的调控途径网络。我们发现,线粒体电子传递链(ETC)成分的表达减少,可以通过不同途径减轻钙失衡和线粒体活性氧的产生,从而防止辣椒素诱导的毒性和其他挑战。此外,我们通过功能获得和功能丧失实验证实了ETC在感觉神经元中的调节作用。有趣的是,与其他感觉神经元亚型相比,TRPV1+感觉神经元维持较低的ETC成分表达,能够更好地耐受兴奋毒性和氧化应激,这意味着ETC调节是一种内在的细胞策略,可以保护伤害感受器免受兴奋毒性的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
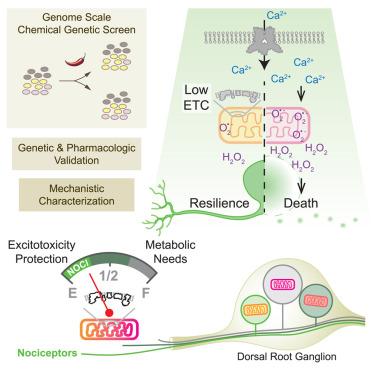
Mitochondrial activity tunes nociceptor resilience to excitotoxicity
The capsaicin receptor, TRPV1, mediates the detection of noxious chemical and thermal stimuli by nociceptors, primary sensory neurons of the pain pathway. Overactivation of TRPV1 leads to cellular damage or death through calcium entry and excitotoxicity. We have exploited this phenomenon to conduct a systematic analysis of excitotoxicity through a genome-wide CRISPRi screen, thereby revealing a comprehensive network of regulatory pathways. We show that decreased expression of mitochondrial electron transport chain (ETC) components protects against capsaicin-induced toxicity and other challenges by mitigating both calcium imbalance and the generation of mitochondrial reactive oxygen species via distinct pathways. Moreover, we confirm the regulatory roles of the ETC in sensory neurons through gain-of-function and loss-of-function experiments. Interestingly, TRPV1+ sensory neurons maintain lower expression of ETC components and can better tolerate excitotoxicity and oxidative stress compared with other sensory neuron subtypes, implicating ETC tuning as an intrinsic cellular strategy that protects nociceptors against excitotoxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


