SATB1是干细胞样CD8+ T细胞静止的关键调节因子
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
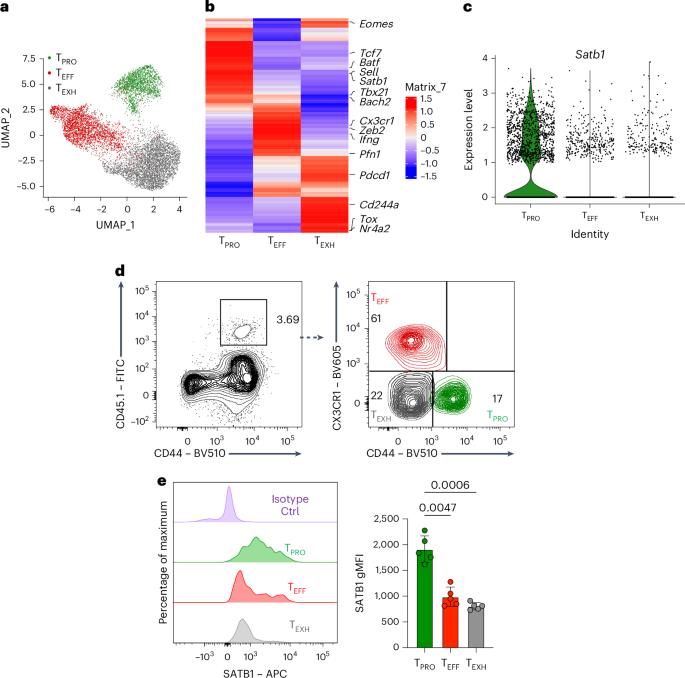
干细胞样祖细胞CD8+ T (TPRO)在慢性感染和癌症期间通过静止、多能和自我更新维持细胞毒性免疫,这些特征与记忆T细胞相同。然而,这些特性如何在持续抗原刺激下保持仍不清楚。在这里,我们发现基因组组织者SATB1选择性地富集在TPRO和记忆CD8+ T细胞中。鉴于其在促进造血干细胞静止中的作用,我们假设SATB1支持CD8+ T细胞的干细胞性。利用CD8+ T细胞特异性CRISPR删除Satb1基因,我们发现Satb1对于慢性淋巴细胞性脉络丛脑膜炎病毒感染期间维持TPRO细胞和急性感染期间记忆CD8+ T细胞形成至关重要。多组学分析显示,SATB1调控包括Tcf7、Bach2和Myb在内的茎秆相关基因的染色质可及性、转录活性和基因组结构。这些发现揭示了SATB1在维持抗原特异性CD8+ T细胞干细胞样状态的转录和表观遗传程序中发挥的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
SATB1 is a key regulator of quiescence in stem-like CD8+ T cells
Stem-like progenitor CD8+ T (TPRO) cells sustain cytotoxic immunity during chronic infection and cancer through quiescence, multipotency and self-renewal, hallmarks shared with memory T cells. However, how these properties are maintained under persistent antigen stimulation remains unclear. Here we identify the genomic organizer SATB1 as selectively enriched in both TPRO and memory CD8+ T cells. Given its role in promoting quiescence in hematopoietic stem cells, we hypothesized that SATB1 supports CD8+ T cell stemness. Using CD8+ T cell-specific CRISPR deletion of the Satb1 gene, we show that SATB1 is essential for maintaining TPRO cells during chronic lymphocytic choriomeningitis virus infection and for memory CD8+ T cell formation during acute infection. Multi-omic profiling revealed that SATB1 regulates the chromatin accessibility, transcriptional activity and genome architecture of stemness-associated genes including Tcf7, Bach2 and Myb. These findings reveal a critical role for SATB1 in preserving the transcriptional and epigenetic programs that sustain the stem-like state of antigen-specific CD8+ T cells. Cui and colleagues identify the chromatin organizer protein SATB1 as a critical regulator of quiescence in stem-like progenitor CD8+ T cells that arise during chronic viral infection and cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


