RETICULATA1是一种定位于质体的碱性氨基酸转运蛋白
IF 13.6
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
植物在提供人体必需的氨基酸方面起着至关重要的作用。20种蛋白质氨基酸中有9种是在质体中完全从头合成的,但介导它们在质体内膜上交换的转运蛋白尚不清楚。在这里,我们发现RETICULATA1 (RE1)是拟南芥中碱性氨基酸(包括Arg、Citr、Orn和lyss)的质体定位转运体。功能丧失突变体显示网状叶表型,含有较低量的碱性氨基酸,并且氨基酸稳态受损。RE1属于一类新的膜转运蛋白,它包含一个功能未知的结构域3411,并且只存在于含有质体的生物体中。我们的结果表明其功能与最接近的同源物RER1重叠,因为双突变体是致命的。同位素标记表明,RE1的损失减少了碱性氨基酸的生物合成,影响了塑质和胞质氨基酸池的平衡。这些发现揭示了质体氨基酸转运体在协调植物初级代谢、发育和营养分配中的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。

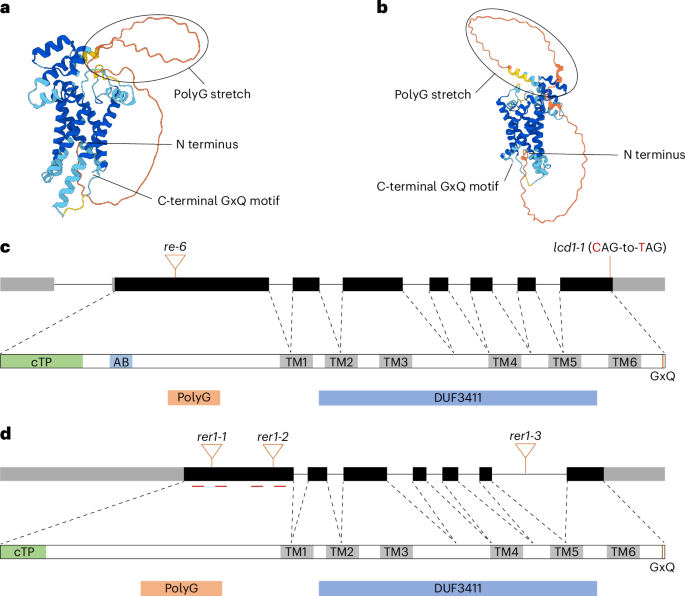
RETICULATA1 is a plastid-localized basic amino acid transporter
Plants have a crucial role in providing essential amino acids for human nutrition. Nine of the 20 proteinogenic amino acids are exclusively synthesized de novo in plastids, yet transporters mediating their exchange across the plastid inner envelope remain unknown. Here we identify RETICULATA1 (RE1) as a plastid-localized transporter for basic amino acids—including Arg, Citr, Orn and Lys—in Arabidopsis thaliana. Loss-of-function mutants display a reticulate leaf phenotype, contain lower amounts of basic amino acids and are impaired in amino acid homeostasis. RE1 belongs to a novel class of membrane transport proteins that contain a domain of unknown function 3411 and are found exclusively in plastid-containing organisms. Our results indicate functional overlap with its closest homologue RER1, as the double mutant is lethal. Isotope labelling reveals that loss of RE1 reduces basic amino acid biosynthesis and affects the equilibration of plastidic and cytosolic amino acid pools. These findings uncover a critical role for plastidial amino acid transporters in coordinating primary metabolism, development and nutrient allocation in plants. RETICULATA1 is a plastid membrane transporter in Arabidopsis that enables basic amino acid exchange across the plastid inner envelope. Loss-of-function mutants reveal its essential role in amino acid homeostasis, plant development and seed production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


