外质体代谢组学揭示糖作为叶肉信使在红光下调节保护细胞离子运输
IF 13.6
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
叶表皮的保护细胞对包围气孔,微观气孔介导CO2吸收和水分流失。历史数据表明,来自叶肉组织内部的信号可能调节气孔开度的保护细胞调节,但任何基于代谢物的信号的分子身份仍然难以捉摸。我们发现拟南芥和蚕豆的细胞外(胞外)液增强了红光诱导的气孔打开。我们广泛的代谢组学分析鉴定了448种外质体代谢物;其中,糖(光合产物)和马来酸在红光下均增加,导致气孔开度增强。免疫组织化学分析显示蔗糖上调H+- atp酶磷酸化,表明atp酶活性增加。膜片钳试验显示,蔗糖抑制缓慢的阴离子电流,从而反对阴离子外流。这些影响发生在与红光下存在的内源性蔗糖浓度相匹配的蔗糖浓度下。这些调节影响促进了保护细胞溶质的输入和保留,从而驱动气孔打开。因此,我们的研究解决了几十年来关于协调光合作用和气孔响应的叶肉信使的存在、身份和机制影响的问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Apoplastic metabolomics reveals sugars as mesophyll messengers regulating guard cell ion transport under red light
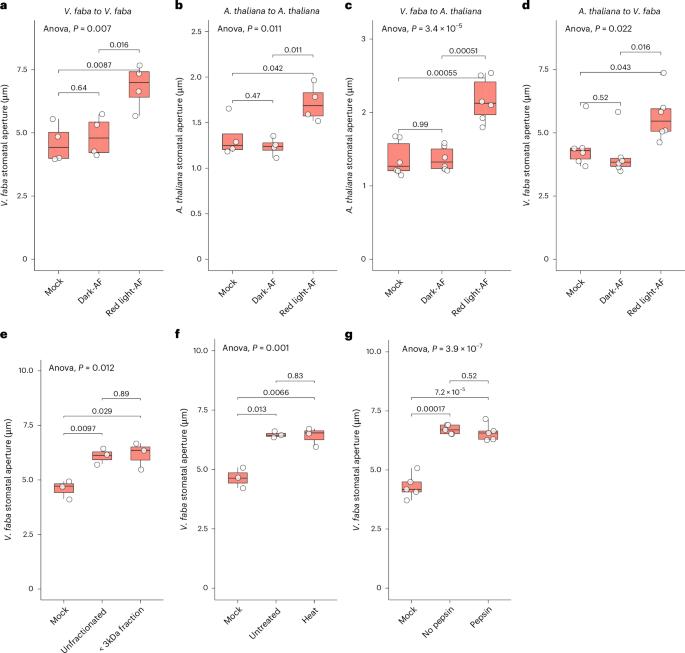
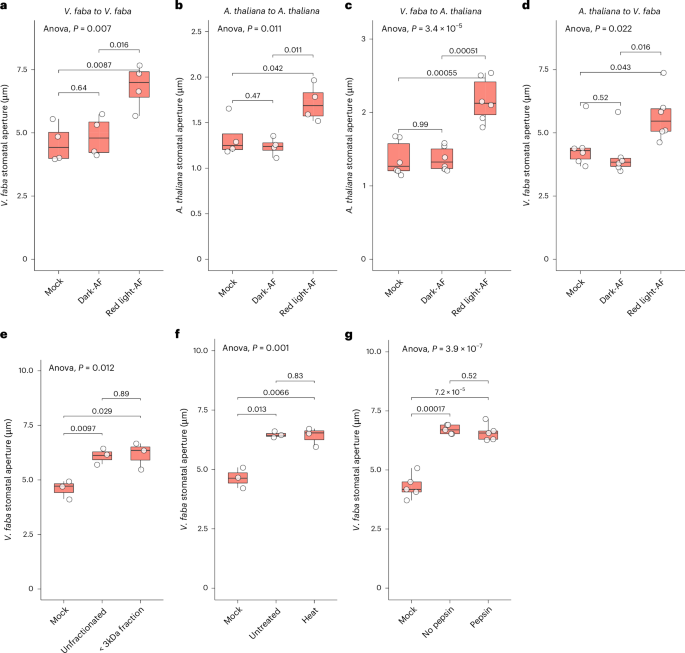
Guard cell pairs in the leaf epidermis enclose stomata, microscopic pores mediating CO2 uptake and water loss. Historical data suggest that signals from interior mesophyll tissue may modulate guard-cell regulation of stomatal apertures, but the molecular identity of any metabolite-based signals has remained elusive. We discovered that extracellular (apoplastic) fluid from Arabidopsis thaliana and Vicia faba enhances red-light-induced stomatal opening. Our extensive metabolomics analyses identified 448 apoplastic metabolites; among these, both sugars (photosynthetic products) and maleic acid increased under red light and caused enhanced stomatal opening. Immunohistochemical assays demonstrated sucrose upregulation of H+-ATPase phosphorylation, indicating increased ATPase activity. Patch clamp assays revealed that sucrose inhibits slow anion currents, thus opposing anion efflux. These impacts occurred at sucrose concentrations matching those present endogenously under red light. These regulatory influences promote guard-cell solute import and retention, which drive stomatal opening. Our research thus addresses the decades-long question concerning the existence, identity and mechanistic impact of mesophyll messengers that coordinate photosynthesis with stomatal response. Guard cells define microscopic stomatal pores for CO2 uptake and water loss. Characterization of the extracellular metabolome revealed sugars as ‘mesophyll messengers’ from the leaf interior that enhance stomatal opening via regulation of the guard-cell H+-ATPase and anion channels.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


