细孔菌肠道定植促进克罗恩病难辨梭菌感染
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
克罗恩病是一种严重的肠道炎症性疾病,目前尚无治愈方法。患有克罗恩病的人患艰难梭菌感染(CDI)的风险增加,这大大加剧了症状。通过一项前瞻性观察性临床研究,结合肠道炎症动物模型,我们发现Veillonella(一种口服共生菌)的肠道定植促进了克罗恩病的CDI。在小鼠中,小叶细络菌抑制主要胆汁酸转运体ASBT的表达,从而阻止胆汁酸的重吸收。同样,细孔菌丰度与克罗恩病患者胆汁酸代谢增加有关。肠道内胆汁酸可用性的增加触发艰难梭菌萌发。小虫草表达一种高度促炎的脂多糖,可触发调节ASBT表达的转录因子c-Jun和c-Fos。这些发现强调,口服共体可加重肠道疾病,为设计治疗克罗恩病患者CDI的治疗方法提供了途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
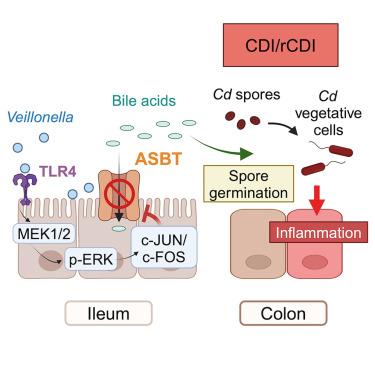
Veillonella intestinal colonization promotes C. difficile infection in Crohn’s disease
Crohn’s disease is a severe inflammatory disorder of the intestine for which there is no cure. Individuals suffering from Crohn’s disease are at an increased risk of developing Clostridioides difficile infection (CDI), which considerably exacerbates symptoms. Using a prospective observational clinical study combined with animal models of intestinal inflammation, we show that intestinal colonization by Veillonella , an oral commensal, promotes CDI in Crohn’s disease. In mice, Veillonella parvula suppresses expression of the main bile acid transporter, ASBT, thus preventing bile acid reabsorption. Similarly, Veillonella abundance is associated with increased bile acid metabolism in Crohn’s disease patients. This increase in bile acid availability within the intestinal lumen triggers C. difficile germination. V. parvula expresses a highly pro-inflammatory lipopolysaccharide that triggers the transcription factors c-Jun and c-Fos regulating ASBT expression. These findings highlight that oral commensals can exacerbate intestinal disease, providing pathways to design therapeutics to treat CDI in Crohn’s disease patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


