具有抗癌和抗菌活性的n -杂环碳硒化合物的合成、DFT及分子对接研究
IF 3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
基于靶标的药物设计是提高候选药物选择性、有效性和安全性的重要策略。本研究设计了苯-咪唑盐(L1-L3)和硒化合物(C1-C3)的合成,考察了它们的酶抑制潜力和生物活性。通过紫外-可见等分析技术证实合成成功。计算(DFT)研究进一步支持了FTIR、1H & 13C NMR和质谱分析。进行C1-C3与关键分子靶点(COX-1、EGF、VEGF、HIF)的分子对接研究。在测试化合物中,C1对EGF的结合亲和力为- 6.14 kcal mol−1,与标准药物5-FU (- 4.97 kcal mol−1)相当。体外对接结果验证证实C1是被试化合物中最有效的抑制剂,对COX-1和EGF的抑制率分别为67.4±1.3%和86.7±1.8%。此外,测试化合物对硫氧还蛋白还原酶(TrxR)具有显著的抑制潜力。对HepG2、HeLa和A-2780细胞株的细胞毒性分析证实C1为先导化合物,IC50值分别为0.956、1.986和0.862 μg/mL。实验化合物对大肠杆菌和金黄色葡萄球菌的抑制范围为8.5±1.1 ~ 27.0±1.2 mm。这些发现表明,基于NHC的硒化合物可能是一种潜在的候选药物,用于化疗多种癌症菌株。本文章由计算机程序翻译,如有差异,请以英文原文为准。
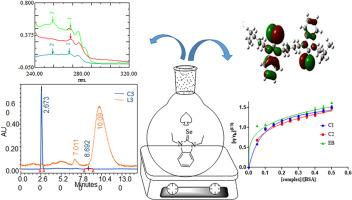
Synthesis, DFT and molecular docking studies of N-Heterocyclic carbene selenium compounds conferring anticancer and antibacterial activity
Target based drug design is an important strategy that increases the selectivity, efficacy and safety of drug candidates. In this study we designed synthesis of benz-imidazolium salts (L1-L3) and selenium compounds (C1–C3) to investigate their enzyme inhibition potential and bioactivity. Successful synthesis was confirmed through analytical techniques like UV–Vis., FTIR, 1H & 13C NMR and mass spectrometry that further supported by computational (DFT) studies. Molecular docking studies of C1–C3 against key molecular targets (COX-1, EGF, VEGF and HIF) was conducted. Among the test compounds C1 showed an impressive binding affinity of −6.14 kcal mol−1 against EGF which is comparable to standard drug 5-FU (−4.97 kcal mol−1). The validation of docking results through in vitro studies confirmed C1 as most potent inhibitor among the test compounds, having inhibition of 67.4 ± 1.3 % and 86.7 ± 1.8 % against COX-1 and EGF respectively. Furthermore, test compounds showed significant inhibition potential against thioredoxin reductase (TrxR). Cytotoxicity profiling across HepG2, HeLa and A-2780 cell lines confirmed C1 as the lead compound, with IC50 values of 0.956, 1.986, and 0.862 μg/mL, respectively. Test compounds also showed antibacterial activity by showing inhibition zone 8.5 ± 1.1–27.0 ± 1.2 mm against E. coli and S. aureus. These findings showed that NHC based selenium compounds could be a potential drug candidate for chemotherapy against multiple cancerous strains.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


