海胆样钴酸镍增强氯析出反应活性
IF 5.7
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
氯析出反应(CER)在化学工业中起着至关重要的作用,然而大多数CER电催化剂依赖于稀有和昂贵的元素,如钌和铱。利用丰富的元素开发高效、可持续、低成本的催化剂是推进生态友好型氯气电化学生产技术的关键。钴酸镍(NiCo₂O₄)因其可用性、可负担性和良好的电化学性能而成为一种有前途的CER催化剂。本研究研究了一种新型的海胆样NiCo₂O₄纳米结构作为催化剂来提高CER性能。电化学分析表明,该纳米结构在10 mA cm-2下的过电位为140 mV, Tafel斜率为69 mV dec -⁻¹,电化学活性表面积为593 cm²。这些发现突出了NiCo₂O₄在氯析出中的优异催化活性、稳定性和效率,为NiCo₂O₄催化剂的合理设计和优化提供了见解,可用于工业过程中的可扩展CER应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
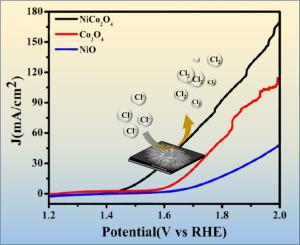
Sea urchin-like nickel cobaltite for enhanced chlorine evolution reaction activity
The chlorine evolution reaction (CER) plays a vital role in the chemical industry, yet most CER electrocatalysts depend on scarce and costly elements like ruthenium and iridium. Developing efficient, sustainable, and cost-effective catalysts using abundant elements is essential to advance eco-friendly electrochemical technologies for chlorine production. Nickel cobaltite (NiCo₂O₄) stands out as a promising CER catalyst due to its availability, affordability, and favourable electrochemical properties. This study investigates a novel sea urchin-like NiCo₂O₄ nanostructure as a catalyst to enhance CER performance. Electrochemical analysis reveals that the nanostructure achieves a low overpotential of 140 mV at 10 mA cm-2, a Tafel slope of 69 mV dec⁻¹, and an electrochemically active surface area of 593 cm². These findings highlight NiCo₂O₄ excellent catalytic activity, stability, and efficiency in chlorine evolution, offering insights for the rational design and optimization of NiCo₂O₄-based catalysts for scalable CER applications in industrial processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Research Bulletin
工程技术-材料科学:综合
CiteScore
9.80
自引率
5.60%
发文量
372
审稿时长
42 days
期刊介绍:
Materials Research Bulletin is an international journal reporting high-impact research on processing-structure-property relationships in functional materials and nanomaterials with interesting electronic, magnetic, optical, thermal, mechanical or catalytic properties. Papers purely on thermodynamics or theoretical calculations (e.g., density functional theory) do not fall within the scope of the journal unless they also demonstrate a clear link to physical properties. Topics covered include functional materials (e.g., dielectrics, pyroelectrics, piezoelectrics, ferroelectrics, relaxors, thermoelectrics, etc.); electrochemistry and solid-state ionics (e.g., photovoltaics, batteries, sensors, and fuel cells); nanomaterials, graphene, and nanocomposites; luminescence and photocatalysis; crystal-structure and defect-structure analysis; novel electronics; non-crystalline solids; flexible electronics; protein-material interactions; and polymeric ion-exchange membranes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


