用1H MAS NMR研究了in改性BEA分子筛上乙烷的H/D交换
IF 4.7
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
在453 ~ 553 K温度下,用1H MAS NMR研究了In改性BEA分子筛(In+/H-BEA和InO+/H-BEA)与纯H型BEA分子筛(H-BEA)的乙烷-d6和br - nsted酸位(BAS)之间的H/D氢交换。动力学测量表明,在H-BEA分子筛上,三种类型的表面OH基团,桥式SiOH、框架外AlOH和强酸性SiOH参与了乙烷-d6在H-BEA分子筛上的交换,反应速率相似。与H- bea和In+/H- bea分子筛上的H/D交换速率和活化能相比,氧化阳离子态的InO+存在于沸石孔隙中时,H/D交换速率显著提高,交换活化能降低。在动力学参数分析的基础上,探讨了in改性沸石交换的可能反应机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
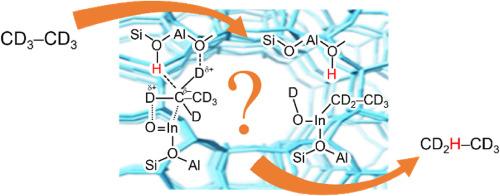
H/D exchange of ethane on In-modified BEA zeolite investigated by 1H MAS NMR
H/D hydrogen exchange between ethane-d6 and Brønsted acid sites (BAS) of In-modified BEA zeolites (In+/H-BEA and InO+/H-BEA) in comparison with the exchange on pure H-form BEA zeolite (H-BEA) has been studied by 1H MAS NMR spectroscopy at 453−553 K. Kinetic measurements show that three types of surface OH groups, the bridged SiOHAl, extra-framework AlOH, and strongly acidic SiOH, are involved in the exchange with ethane-d6 on H-BEA zeolite with similar reaction rates. In case of the presence of oxo-cationic InO+ species in zeolite pores, the rate of H/D exchange increases significantly and the activation energy of the exchange decreases as compared to the rate and the activation energy on H-BEA and In+/H-BEA zeolites. The possible reaction mechanisms of the exchange for In-modified zeolites are discussed based on the analysis of the kinetic parameters.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


