黑曲霉中未描述的烯丙化蒽醌类化合物。Bornm交货。具有细胞毒性
IF 2.6
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
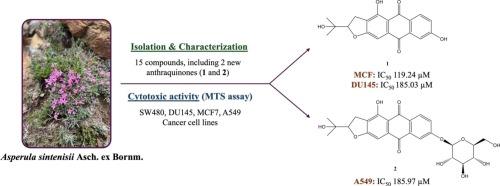
从曲霉MeOH提取物中分离到两种蒽醌类化合物,分别命名为sinintenisiquinones A(1)和B(2),并分离到五种环烯醚萜苷、五种黄酮类化合物和三种酚酸衍生物。它们的化学结构通过广泛的1D (1H和13C)和2D (COSY, HSQC和HMBC) NMR波谱和HRESIMS进行了鉴定。化合物1和2是一种罕见的蒽醌衍生物,其戊烯基环化成二氢呋喃环。采用MTS法对选定化合物(1 - 3,5,7,11,12和15)对SW480, DU145, MCF7和A549癌细胞以及健康细胞系L929的体外细胞毒活性进行了评估。与阳性对照阿霉素相比,化合物1和2表现出较弱的细胞毒性。本研究首次对我国本文章由计算机程序翻译,如有差异,请以英文原文为准。
Undescribed prenylated anthraquinones from Asperula sintenisii Asch. Ex Bornm. with cytotoxic activities
Two previously unreported anthraquinones, named sintenisiquinones A (1) and B (2), were isolated from the MeOH extract of Asperula sintenisii together with five iridoid glycosides, five flavonoids, as well as three phenolic acid derivatives. Their chemical structures were elucidated on the basis of extensive 1D (1H and 13C) and 2D (COSY, HSQC, and HMBC) NMR spectroscopy and HRESIMS. Compounds 1 and 2 represent rare type of anthraquinone derivatives bearing a prenyl group cyclized to dihydrofuran ring. The in vitro cytotoxic activities of selected compounds (1–3, 5, 7, 11, 12 and 15) were evaluated against SW480, DU145, MCF7, and A549 cancer cell lines as well as a healthy cell line, L929 by MTS assay. Compounds 1 and 2 displayed weak cytotoxicity compared to positive control, doxorubicin. This is the first phytochemical and bioactivity studies on A. sintenisii, an endemic species to Türkiye.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


