二芳基方胺和二芳基马来酰亚胺的合成
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
3,4-二芳基-1,2-平方炔(3,4-二芳基环丁-3-烯-1,2-二酮)和3,4-二芳基马来酰亚胺(3,4-二芳基- 1h -吡咯-2,5-二酮)是强电子受体,可以与富电子芳烃配对,形成简单而强大的给受体单体,进一步发展为有效的共轭聚合物。采用类似friedel - crafts取代的方法,得到了一系列的1,2-squaraines的共轭3,4-二噻吩基衍生物,收率可达96%。刚实现了方胺核向类似的电子接受马来酰亚胺的简单一步转化,使方胺能高效地从方胺中获得二芳基马来酰亚胺。提出了一种可能的电环开/再闭后氧化转化机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
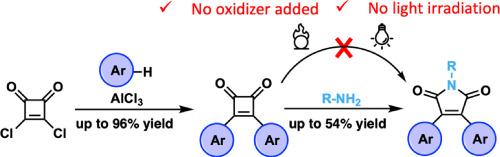
Synthesis of diaryl squaraines and diaryl maleimides
The 3,4-diaryl-1,2-squaraines (3,4-diaryl cyclobut-3-ene-1,2-diones) and 3,4-diaryl maleimides (3,4-diaryl-1H-pyrrole-2,5-diones) are strong electron acceptors that can be paired with electron rich aromatics to create simple but powerful donor-acceptor monomeric units to be further developed toward effective conjugated polymers. A series of conjugated 3,4-dithienyl derivatives of 1,2-squaraines were obtained from Friedel-Crafts-like substitution protocol in good to excellent yields (up to 96 %). The simple one-step conversion of squaraine core to the analogous electron-accepting maleimides were just realized, which led to efficient access of diaryl maleimides from squaraines. The possible electrocyclic ring opening/re-closing followed by oxidation mechanism was proposed for the transformation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


