新型二异丁基二胺接枝聚合物树脂从轻度硝酸介质中回收铀
IF 4.8
2区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
摘要
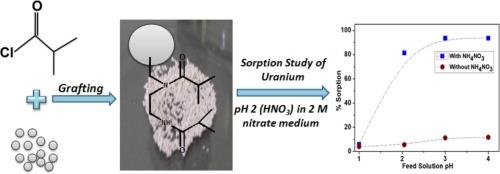
将二异丁基二胺(DIBDA)接枝树脂与市售苯乙烯-二乙烯基苯聚合物树脂进行化学接枝,合成了一种新型的DIBDA接枝树脂。采用FTIR,固态13-C CP/MAS NMR,热重分析(TGA),扫描电子显微镜(SEM)和能量色散光谱(EDS)等标准技术对树脂进行表征。在批式和柱式两种模式下系统地评价了铀和竞争金属离子的吸附行为。在pH为2-3的稀硝酸介质中,在2.0 M硝酸离子的存在下,获得了最佳的铀吸收率。在0.25 M HNO3的条件下,一次有效地剥离了98%以上的铀。柱式研究使用含有0.1 g/L铀的进料和6 mL树脂床体积,得到铀浓度超过1.2 g/L的条状溶液,对应于浓度系数大于12。该树脂对铀的选择性优于普通基体元素,如Fe、Y、Ca、Al和Na。动力学研究和吸附等温线模型揭示了酰胺官能团对U(VI)的选择性和高效吸附的重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Novel diisobutyl diamide grafted polymer resin for uranium recovery from mild nitric acid medium
A novel diisobutyl diamide (DIBDA) grafted resin was synthesized by chemically grafting the amidic functionalities onto a commercially available styrene-divinylbenzene polymer resin. The resin was characterized using standard techniques such as FTIR, solid-state 13-C CP/MAS NMR, thermogravimetric analysis (TGA), scanning electron microscopy (SEM), and energy-dispersive spectroscopy (EDS). The sorption behavior of uranium and competing metal ions was systematically evaluated in both batch and column modes. Optimal uranium uptake was achieved from a dilute nitric acid medium at pH 2–3 in the presence of 2.0 M nitrate ions. More than 98 % uranium was stripped efficiently in a single step using 0.25 M HNO3. Column studies using a feed containing 0.1 g/L U and a 6 mL resin bed volume yielded a strip solution with uranium concentration exceeding 1.2 g/L, corresponding to a concentration factor above 12. The resin exhibited excellent selectivity for uranium over common matrix elements such as Fe, Y, Ca, Al, and Na. Kinetic studies and sorption isotherm modelling revealed the significant role of amidic functional groups in the selective and efficient sorption of U(VI).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Hydrometallurgy
工程技术-冶金工程
CiteScore
9.50
自引率
6.40%
发文量
144
审稿时长
3.4 months
期刊介绍:
Hydrometallurgy aims to compile studies on novel processes, process design, chemistry, modelling, control, economics and interfaces between unit operations, and to provide a forum for discussions on case histories and operational difficulties.
Topics covered include: leaching of metal values by chemical reagents or bacterial action at ambient or elevated pressures and temperatures; separation of solids from leach liquors; removal of impurities and recovery of metal values by precipitation, ion exchange, solvent extraction, gaseous reduction, cementation, electro-winning and electro-refining; pre-treatment of ores by roasting or chemical treatments such as halogenation or reduction; recycling of reagents and treatment of effluents.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


