包裹MSC-Exos和ZIF-8的水凝胶支架通过协调成骨和免疫调节促进骨再生
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
有限的骨再生和不理想的免疫反应是修复临界尺寸骨缺损的主要挑战。间充质干细胞衍生外泌体(MSC-Exos)作为一种新兴的治疗方式,在骨再生中具有广阔的应用前景。在这项研究中,骨功能化的MSC-Exos被装载到用骨免疫调节剂沸石咪唑酸框架-8 (ZIF-8)修饰的GelMA水凝胶支架中,用于修复临界尺寸的骨缺损。制备的MSC-Exos/ZIF-8@GelMA复合水凝胶具有良好的生物相容性,有利于骨髓间充质干细胞(BMSCs)的粘附和增殖。外泌体和锌离子的持续释放使复合水凝胶具有协同增强骨再生、血管生成和免疫调节的作用。具体来说,内化MSC-Exos中的microRNA-23a-3p通过靶向PTEN节点激活BMSCs中的AKT信号通路,上调成骨相关标志物的表达。同时,首次证实在模拟炎症条件下,ZIF-8抑制RAW264.7细胞非经典NF-κB通路的激活,从而下调促炎细胞因子的表达,诱导m2型极化。大鼠颅骨骨缺损模型表明,复合水凝胶在体内可显著促进新生骨形成和血管生成,并维持低水平的炎症。复合水凝胶具有增强成骨和免疫调节的协同作用,是开发骨组织工程支架的一种新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
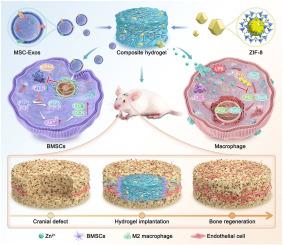
Hydrogel scaffold encapsulating MSC-Exos and ZIF-8 promotes bone regeneration via coordinating osteogenesis and immunomodulation
The limited bone regeneration and suboptimal immune responses constitute the major challenges in repairing critical-sized bone defects. As an emerging therapeutic modality, mesenchymal stem cell-derived exosomes (MSC-Exos) exhibit promising application prospects in bone regeneration. In this study, the bone-functionalized MSC-Exos are loaded into GelMA hydrogel scaffolds modified with the bone immunomodulator Zeolitic Imidazolate Framework-8 (ZIF-8) for the repair of critical-sized bone defects. The prepared MSC-Exos/ZIF-8@GelMA composite hydrogel demonstrates excellent biocompatibility and favors the adhesion and proliferation of bone marrow mesenchymal stem cells (BMSCs). The sustained release of exosomes and zinc ions endows the composite hydrogel with synergistically enhanced bone regeneration, angiogenesis, and immunomodulation. Specifically, the microRNA-23a-3p within internalized MSC-Exos activates the AKT signaling pathway in BMSCs by targeting the PTEN node and up-regulates the expression of osteogenesis-related markers. Meanwhile, it is demonstrated for the first time that ZIF-8 inhibits the activation of the non-classical NF-κB pathway in RAW264.7 cells under simulated inflammatory conditions, thereby downregulating pro-inflammatory cytokine expression and inducing M2-type polarization. The rat cranial bone defect model demonstrates that the composite hydrogel significantly enhances new bone formation and angiogenesis in vivo and maintains a low level of inflammation. The design of a composite hydrogel featuring synergistic enhancement of osteogenesis and immunomodulation represents a novel strategy for developing bone tissue engineering scaffolds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


