苯并噻吩亚胺海湾区的硝化取向:N环和s环衍生物的流线型途径
IF 4.2
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
n -环化最近成为一种调制苯并噻吩亚胺(BTI)衍生物光电特性的有效方法,使其能够在从发光器件到光动力治疗等领域得到应用。在这项研究中,我们报告了一种优化的合成策略,将n-环状bti的原始七步工艺减少到只有四个步骤,效率提高了约40%。此外,我们引入了一种新的s环BTI衍生物,扩展了涉及杂原子的环融合的范围。通过对N环和s环化合物的对比分析,揭示了单原子修饰对π共轭体系的显著影响。这些发现证明了环化作为一种化学上可接近的多功能工具的效用,可以微调具有定制光物理性质的分子结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
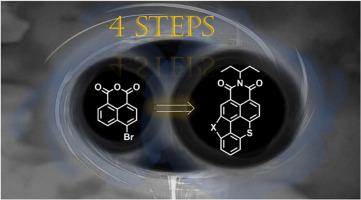
Orientation of nitration in bay-region of benzothioxanthene imide: A streamlined pathway to N- and S-annulated derivatives
N-annulation has recently emerged as a powerful approach for modulating the optoelectronic properties of benzothioxanthene imide (BTI) derivatives, enabling their application in areas ranging from light-emitting devices to photodynamic therapy. In this study, we report an optimized synthetic strategy that reduces the original seven-step process for N-annulated BTIs to just four steps, achieving a ∼40 % improvement in efficiency. Additionally, we introduce a novel S-annulated BTI derivative, extending the scope of heteroatom-involving ring fusion. Comparative analysis between N- and S-annulated compounds reveals the pronounced influence of a single-atom modification on the π-conjugated system. These findings demonstrate the utility of annulation as a chemically accessible and versatile tool for fine-tuning molecular architectures with tailored photophysical properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


