msc来源的细胞外小泡通过miR-29a-3p调节的小胶质细胞m1样极化减轻糖尿病视网膜病变
IF 2.7
2区 医学
Q1 OPHTHALMOLOGY
引用次数: 0
摘要
糖尿病性视网膜病变(DR)被认为是一种神经血管疾病,在全世界的工作年龄成年人中造成永久性视力丧失和失明。间充质干细胞(MSC)衍生的小细胞外囊泡(sev)是炎症调节的一个有吸引力的候选者。然而,MSCs分泌的sev对糖尿病视网膜病变小胶质细胞M1分化的调控作用尚未得到充分研究。在本研究中,通过HMGB1/TLR4信号通路,玻璃体内注射sev可减少视网膜炎症,减轻血管渗漏,抑制m1样小胶质细胞。基于msc - sev miRNA测序、生物信息学预测和双荧光素酶报告基因测定,miR-29a-3p被确定为通过下调HMGB1调控m1样小胶质细胞的关键效应物。miR-29a-3p在msc - sev中的沉默使其对stz诱导的糖尿病大鼠和晚期糖基化终产物(AGEs)处理的人小胶质细胞(HMC3)的治疗效果失效。在msc - sev中沉默miR-29a-3p逆转了msc - sev对stz诱导的大鼠和晚期糖基化终产物(AGEs)治疗的HMC3的治疗作用。此外,过表达miR-29a-3p可以抑制m1样小胶质细胞,过表达HMGB1可以有效逆转这种抑制。总体而言,本研究表明,携带miR-29a-3p的msc - sev通过靶向HMGB1减少M1小胶质细胞极化,从而减轻糖尿病大鼠的视网膜损伤,从而减少炎症并保护血视网膜屏障(BRB)。msc - sev和mirna可能是DR的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
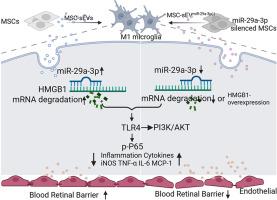
MSC-derived small extracellular vesicles attenuate diabetic retinopathy through miR-29a-3p regulated microglia M1-like polarization
Diabetic retinopathy (DR), considered as a neurovascular disorder, significantly causes permanent vision loss and blindness worldwide among working-age adults. The inflammation caused by M1-like microglia is involved in DR. Mesenchymal stem cell (MSC)-derived small extracellular vesicles (sEVs) is an attractive candidate for inflammation modulation. However, the regulatory effect of sEVs secreted by MSCs on M1 differentiation of microglia in diabetic retinopathy has not been thoroughly investigated. In this study, intravitreal injection of sEVs reduced retinal inflammation, mitigated vascular leakage, and suppressed M1-like microglia via the HMGB1/TLR4 signaling pathway. Based on MSC-sEVs miRNA sequencing, bioinformatics prediction, and dual-luciferase reporter assay, miR-29a-3p was identified as a key effector in the modulation of M1-like microglia through the down-regulation of HMGB1. The silencing of miR-29a-3p in MSC-sEVs negated their therapeutic efficacy in STZ-induced diabetic rats and human microglial cells (HMC3) treated with advanced glycation end products (AGEs). Silencing miR-29a-3p in MSC-sEVs reversed the therapeutic effects of MSC-sEVs on STZ-induced rats and advanced glycation end products (AGEs)-treated HMC3. Additionally, overexpression of miR-29a-3p could suppress M1-like microglia, which could be effectively reversed by overexpressing HMGB1. Overall, this study demonstrated that MSC-sEVs carrying miR-29a-3p attenuate retinal injury in diabetic rats by reducing M1 microglia polarization through the targeting of HMGB1, thereby reducing inflammation and protecting the blood-retinal barrier (BRB). MSC-sEVs and miRNAs may be explored as promising therapeutic targets for DR.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental eye research
医学-眼科学
CiteScore
6.80
自引率
5.90%
发文量
323
审稿时长
66 days
期刊介绍:
The primary goal of Experimental Eye Research is to publish original research papers on all aspects of experimental biology of the eye and ocular tissues that seek to define the mechanisms of normal function and/or disease. Studies of ocular tissues that encompass the disciplines of cell biology, developmental biology, genetics, molecular biology, physiology, biochemistry, biophysics, immunology or microbiology are most welcomed. Manuscripts that are purely clinical or in a surgical area of ophthalmology are not appropriate for submission to Experimental Eye Research and if received will be returned without review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


