电解液电导率对锂离子电池性能的影响:采用对称电池的电化学阻抗分析
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
锂离子电池具有能量密度高、循环寿命长、效率高等优点,在储能领域得到了广泛的应用。电解质的选择对圆柱形锂离子电池的电化学性能有显著影响。本研究考察了四种不同的电解质——双(氟磺酰)亚胺锂(LiFSI)、六氟磷酸锂(LiPF6)、双(五氟乙基磺酰)亚胺锂(LiBETI)和四氟硼酸锂(LiBF4)对电池性能的影响,评估了它们对电导率、电阻成分和整体电化学行为的影响。使用评价电池测量的电解质的离子电导率受到阴离子种类的显著影响,在测试的电解质中,LiFSI的电导率最高。此外,利用对称池进行了电化学阻抗谱分析,以确定各种电阻分量的贡献。结果表明,LiFSI的电导率最高,电阻最低,其次是LiPF6和LiBETI, LiBF4的电导率最差。在较宽的温度范围内测量了电极内离子电导率与电解质离子电导率的关系,并对电极的弯曲系数进行了评价。电解质的离子电导率与电极中的离子电导率之间的关系在不同的电解质中是一致的。此外,利用电解质的离子电导率和电极的弯曲系数确定电极中的离子电阻。在所研究的电解质中,LiFSI表现出最高的离子电导率,从而降低了电极中的离子电阻,提高了电池性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
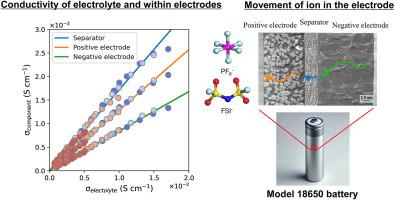
Influence of electrolyte conductivity on the performance of lithium-ion batteries: An electrochemical impedance analysis using symmetric cells
Lithium-ion batteries are widely used in energy storage applications owing to their high energy density, long cycle life, and efficiency. The electrochemical performance of cylindrical lithium-ion batteries is significantly influenced by the choice of electrolytes. This study examines the impact of four different electrolytes-lithium bis (fluor sulfonyl) imide (LiFSI), lithium hexafluorophosphate (LiPF6), lithium bis (pentafluoroethanesulfonyl) imide (LiBETI), and lithium tetrafluoroborate (LiBF4)-on the battery performance by evaluating their influence on the conductivity, resistance components, and overall electrochemical behavior. The ionic conductivity of the electrolytes, measured using evaluation cells, is significantly influenced by the anionic species, with LiFSI exhibiting the highest conductivity among the tested electrolytes. Additionally, electrochemical impedance spectroscopy was performed using symmetric cells to determine the contributions of various resistance components. The results indicate that LiFSI exhibited the highest conductivity and lowest resistance, followed by LiPF6 and LiBETI, with LiBF4 exhibiting the poorest performance. The relationship between the ionic conductivity within the electrode and the ionic conductivity of the electrolytes are measured over a wide temperature range, and the tortuosity factors of the electrodes are evaluated. The relationship between the ionic conductivity of the electrolyte and the ionic conductivity in the electrode is consistent across different electrolytes. Furthermore, the ionic resistance in the electrode is determined using the ionic conductivity of the electrolyte and the tortuosity factor of the electrode. Among the studied electrolytes, LiFSI demonstrated the highest ionic conductivity, leading to reduced ionic resistance in the electrode and improved battery performance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


