利用常压MALDI技术对RNA消化产物进行高通量mRNA序列确认的概念
IF 1.7
3区 化学
Q3 PHYSICS, ATOMIC, MOLECULAR & CHEMICAL
引用次数: 0
摘要
mRNA疫苗的开发和生产需要经常采取质量控制措施,以确保产品的安全性和有效性。质谱与液相色谱(LC-MS)相结合被认为是序列确认和转录后修饰评估的有力工具。LC-MS方法虽然准确,但采集时间长,因此通常不适合高通量应用。本研究介绍了一种利用常压UV-MALDI (AP-MALDI)结合高分辨率精确质量(HRAM) Orbitrap™质量分析仪技术进行RNA寡核苷酸序列确认的新方法。通过使用Orbitrap检测器,我们实现了RNA消化产物的质量精度低于2 ppm,适用于验证复杂混合物中的寡核苷酸序列,而无需事先分离。我们使用具有g特异性裂解的RNase T1,一种氨活化阳离子交换树脂进行纯化,然后进行AP-UV-MALDI质谱分析。用合成的119mer RNA寡核苷酸验证了该方法,并通过MS/MS在正离子和负离子模式下确认了序列的一致性。该方法进一步应用于疫苗变体mRNA序列的计算机分析,展示了其在高通量、快速序列确认和RNA变异分化方面的潜力,尤其与生物制药制造中的mRNA疫苗开发和质量控制相关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
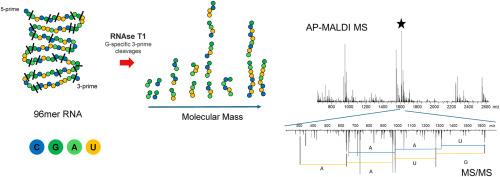
Concept for high-throughput mRNA sequence confirmation by atmospheric pressure MALDI technique applied to RNA digestion products
Development and manufacturing of mRNA vaccines require frequent quality control measures to ensure product safety and efficacy. Mass Spectrometry in combination with Liquid Chromatography (LC-MS) is known as a powerful tool for sequence confirmation and assessment of post-transcriptional modifications. LC-MS methods, while accurate, suffer from long acquisition times, making them typically impractical for high-throughput applications.
This study introduces a novel approach for RNA oligonucleotide sequence confirmation using UV-MALDI at atmospheric pressure (AP-MALDI) coupled with high-resolution accurate mass (HRAM) Orbitrap™ mass analyzer technology. By using an Orbitrap detector, we achieve a mass accuracy below 2 ppm for the digestion products of a RNA digest, suitable for verifying oligonucleotide sequences from complex mixtures without prior separation. We employ RNase T1 owing its G-specific cleavage, an ammonium-activated cation exchange resin for purification, followed by AP-UV-MALDI MS analysis. The method is validated with a synthetic 119mer RNA oligonucleotide, confirming sequence identity through MS/MS in both positive and negative ion modes. The approach is further applied to in silico analysis of mRNA sequences from vaccine variants, showcasing its potential for high-throughput, rapid sequence confirmation, and variant differentiation in RNA, particularly relevant for mRNA vaccine development and quality control in biopharmaceutical manufacturing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.60
自引率
5.60%
发文量
145
审稿时长
71 days
期刊介绍:
The journal invites papers that advance the field of mass spectrometry by exploring fundamental aspects of ion processes using both the experimental and theoretical approaches, developing new instrumentation and experimental strategies for chemical analysis using mass spectrometry, developing new computational strategies for data interpretation and integration, reporting new applications of mass spectrometry and hyphenated techniques in biology, chemistry, geology, and physics.
Papers, in which standard mass spectrometry techniques are used for analysis will not be considered.
IJMS publishes full-length articles, short communications, reviews, and feature articles including young scientist features.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


