RNA纳米颗粒基因治疗对Twist1突变小鼠颅缝闭合和早期缝合融合的抑制作用
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
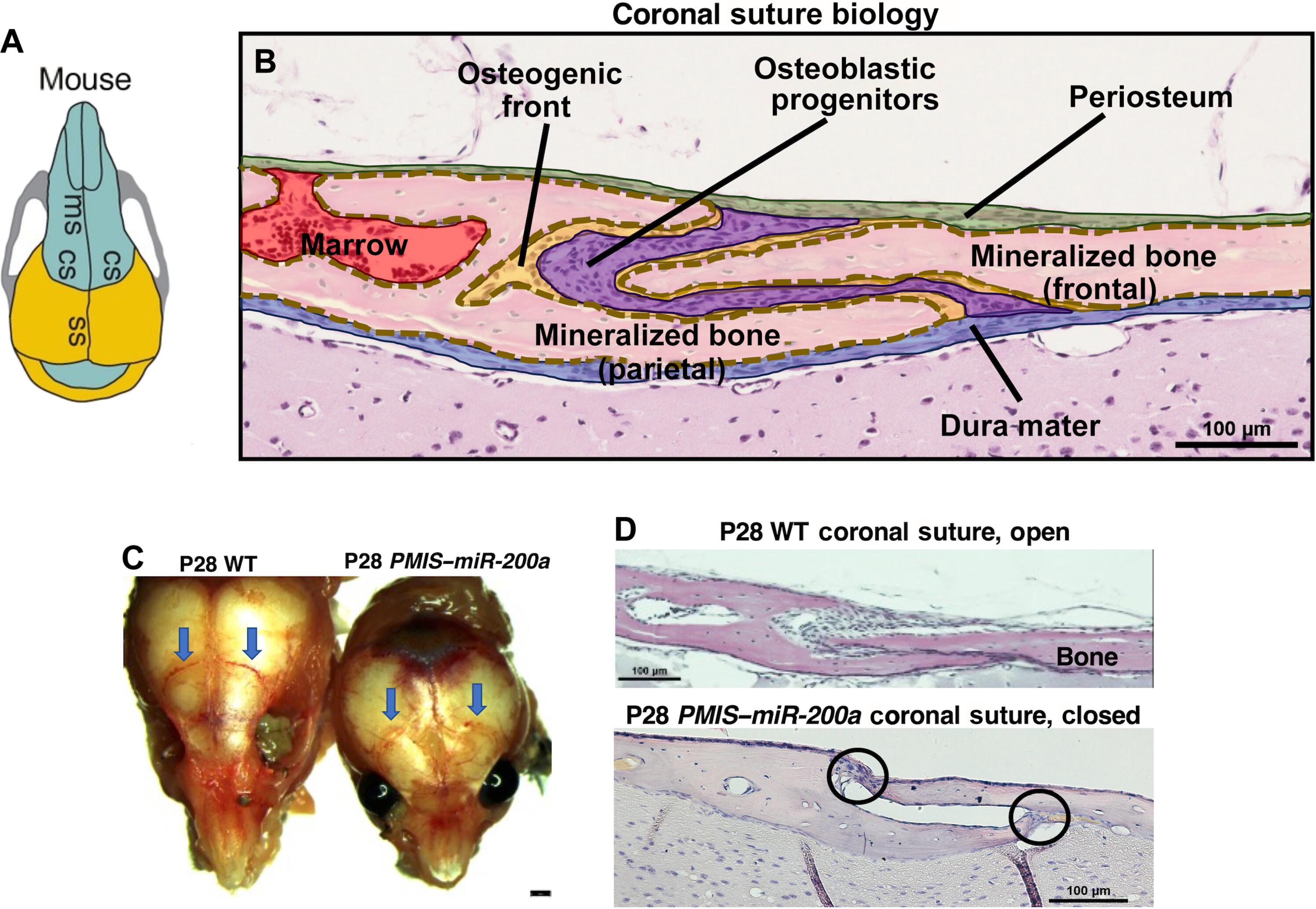
颅缝闭锁是一种常见的出生缺陷,每2200个活产儿中就有1个因颅缝过早融合而导致严重的颅骨和认知缺陷。目前的手术治疗需要对婴儿进行侵入性颅拱顶重塑和颅骨切除术。我们证明抑制miR-200a在PMIS-miR-200a小鼠中导致冠状缝合线融合(颅缝闭合)。因此,我们使用过表达miR-200a来阻止Twist1突变小鼠(一种众所周知的颅缝闭合模型)的缝合线融合。我们开发了一种聚链化肽纳米颗粒系统,在缝合融合前将表达miR-200a的质粒DNA直接递送到出生后第4天(P4) Twist1突变小鼠的缝合线中。P7 ~ P10缝合融合前在头皮下注射miR-200a纳米颗粒抑制缝合融合。处理增加了Gli1和six2阳性缝合干细胞和骨膜层厚度。经扭1+/−处理的小鼠体重增加,警觉和活跃。我们展示了一种有效的无创基因治疗颅缝闭锁的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Inhibition of craniosynostosis and premature suture fusion in Twist1 mutant mice with RNA nanoparticle gene therapy
Craniosynostosis is a common birth defect affecting 1 of the 2200 live births causing severe skull and cognitive defects, due to premature cranial suture fusion. The current surgical treatments require invasive calvaria vault remodeling and cranial bone resection in the baby. We demonstrate that inhibition of miR-200a in PMIS–miR-200a mice results in coronal suture fusion (craniosynostosis). Therefore, we use overexpression of miR-200a to prevent suture fusion in Twist1 mutant mice, a well-known model for craniosynostosis. We developed a PEGylated-peptide nanoparticle system to deliver plasmid DNA expressing miR-200a directly to the sutures of postnatal day 4 (P4) Twist1 mutant mice before suture fusion. Injection of the miR-200a nanoparticles under the scalp before suture fusion at P7 to P10 inhibited suture fusion. Treatments increased Gli1- and Six2-positive suture stem cells and the thickness of the periosteum layer. The treated Twist1+/− mice increased body weight and were alert and active. We demonstrate an effective noninvasive gene therapy treatment for craniosynostosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


