强效AMPK抑制剂BAY-3827的机制和细胞作用
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
腺苷5 ' -单磷酸腺苷(AMP)活化蛋白激酶(AMPK)的抑制作用因其在许多疾病中的治疗潜力而受到越来越多的研究。然而,现有的AMPK抑制剂有限,选择性差,脱靶效应大。在这里,我们提供了机制的见解,并描述了最近发现的AMPK抑制剂BAY-3827的细胞选择性。含有BAY-3827的AMPK激酶结构域的2.5-Å共晶结构显示出明显的特征,包括αD螺旋Cys106和激活环残基Cys174之间的二硫桥。这个桥似乎稳定了激活环,使得Asn162将asp - ph - gly (DFG)基序Phe158重新定位到c端叶,取代His137并破坏调控脊柱,促进激酶的失活状态。在肝细胞中,BAY-3827阻断AMPK激活因子(MK-8722)介导的ACC1磷酸化和相应的脂肪生成抑制。转录组分析显示,BAY-3827下调了约30%受mk -8722刺激的ampk依赖基因。我们建立了BAY-3827的选择性和效用的分子和细胞基础,以描绘AMPK功能,同时强调其局限性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
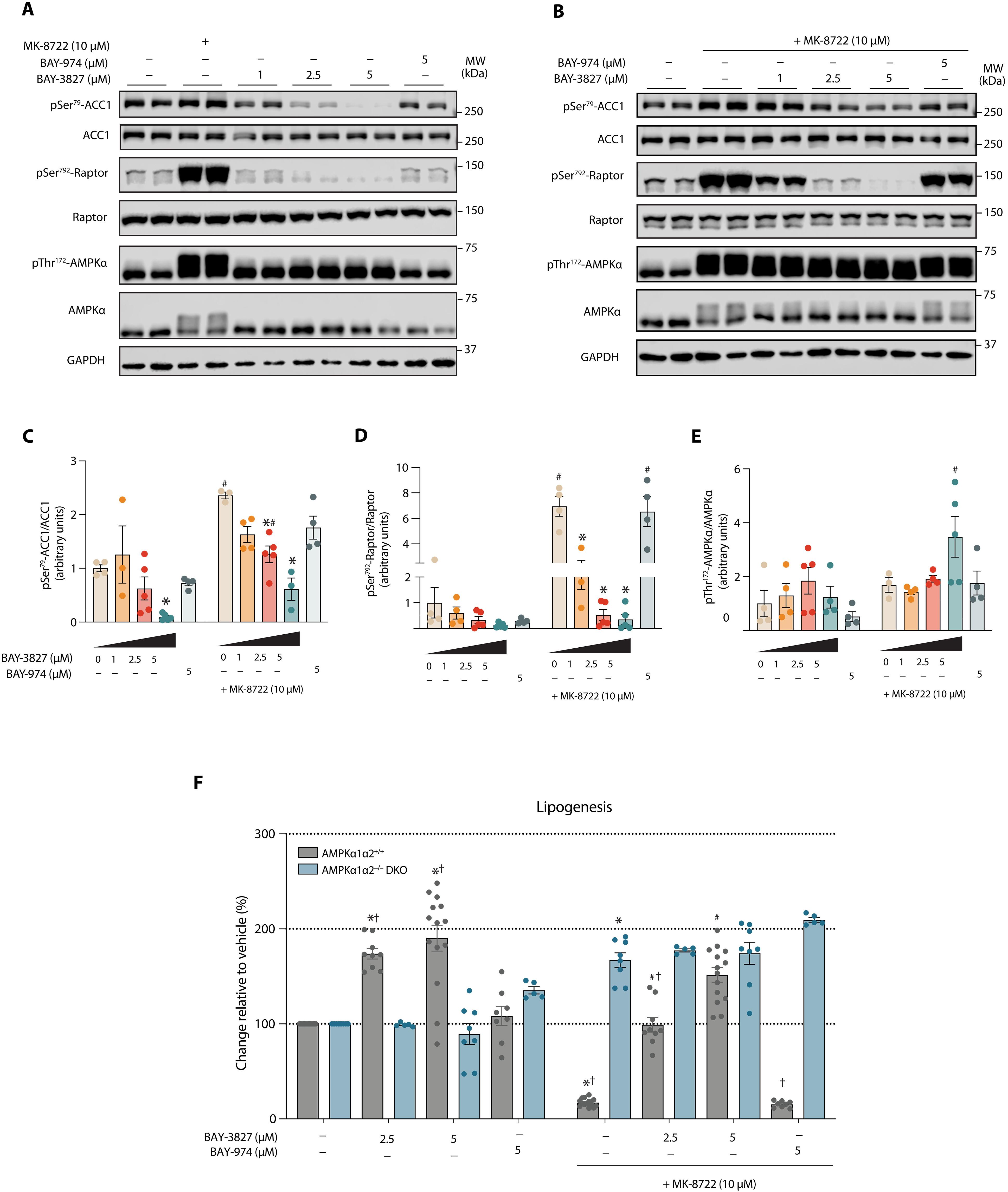
Mechanism and cellular actions of the potent AMPK inhibitor BAY-3827
Inhibition of adenosine 5′-monophosphate (AMP)–activated protein kinase (AMPK) is under increasing investigation for its therapeutic potential in many diseases. Existing AMPK inhibitors are however limited, with poor selectivity and substantial off-target effects. Here, we provide mechanistic insights and describe the cellular selectivity of the recently identified AMPK inhibitor BAY-3827. A 2.5-Å cocrystal structure of the AMPK kinase domain with BAY-3827 revealed distinct features including a disulfide bridge between the αD helix Cys106 and the activation loop residue Cys174. This bridge appears to stabilize the activation loop such that Asn162 repositions the Asp-Phe-Gly (DFG) motif Phe158 toward the C-terminal lobe, displacing His137 and disrupting the regulatory spine, promoting an inactive kinase state. In hepatocytes, BAY-3827 blocked AMPK activator (MK-8722)–mediated phosphorylation of ACC1 and corresponding inhibition of lipogenesis. Transcriptome analysis revealed that BAY-3827 down-regulated ~30% of MK-8722–stimulated AMPK-dependent genes. We establish the molecular and cellular basis of BAY-3827’s selectivity and utility for delineating AMPK functions while highlighting its limitations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


