基于荧光素酶的快速稳健报告系统评估SARS-CoV-2蛋白酶活性
IF 2.4
3区 医学
Q3 VIROLOGY
引用次数: 0
摘要
尽管已经出现了有效的抗病毒药物来对抗SARS-CoV-2感染,但需要新的治疗策略来更好地应对病毒正在进行和未来的演变。靶向病毒蛋白酶,如主蛋白酶(Mpro),仍然是一种有前途的方法。在这里,我们提出了一个快速和敏感的基于发光的报告系统,i-NSP4/5-Gluc2,来评估Mpro的活性。该系统采用高斯荧光素酶(Gluc)融合到含有特定Mpro切割位点的前白细胞介素1β (il -1β)片段。在Mpro分裂后,Gluc被释放和分泌,在细胞外产生发光信号。通过优化系统设计和实验条件,获得了较高的灵敏度和特异度。i-NSP4/5-Gluc2系统使用Mpro抑制剂Nirmatrelvir进行了验证,并成功地从46个化合物的小文库中鉴定出潜在的Mpro抑制剂,作为概念验证。值得注意的是,在i-NSP4/5-Gluc2试验鉴定的14种新化合物中,有13种对活的SARS-CoV-2表现出有效的抗病毒活性,这突出了该系统的准确性和预测能力。这种与bsl2兼容的高通量方法有助于快速有效地筛选抗病毒化合物,加速开发针对SARS-CoV-2和未来病毒大流行的有效治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
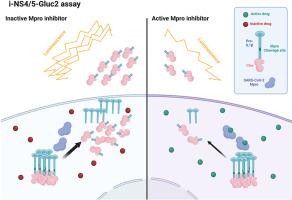
A rapid and robust luciferase-based reporter system to assess SARS-CoV-2 protease activity
Despite effective antiviral drugs that have emerged to combat SARS-CoV-2 infections, novel therapeutic strategies are required to better address the ongoing and future evolutions of the virus. Targeting viral proteases, such as the main protease (Mpro), remains a promising approach. Here, we present a rapid and sensitive luminescence-based reporter system, the i-NSP4/5-Gluc2, to assess Mpro activity. This system employs Gaussia luciferase (Gluc) fused to a pro-interleukin 1β (pro-IL-1β) fragment containing a specific Mpro cleavage site. Upon Mpro cleavage, Gluc is released and secreted, generating a luminescent signal outside the cells. By optimizing the system's design and experimental conditions, we achieved high sensitivity and specificity. The i-NSP4/5-Gluc2 system was validated using the Mpro inhibitor Nirmatrelvir and successfully identified potential Mpro inhibitors from a small library of 46 compounds, as proof of concept. Notably, 13 out of 14 new compounds identified by the i-NSP4/5-Gluc2 assay exhibited potent antiviral activity against live SARS-CoV-2, highlighting the system's accuracy and predictive power. This BSL2-compatible, high-throughput approach facilitates rapid and efficient screening of antiviral compounds, accelerating the development of effective therapeutics against SARS-CoV-2 and future viral pandemics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Virology
医学-病毒学
CiteScore
6.00
自引率
0.00%
发文量
157
审稿时长
50 days
期刊介绍:
Launched in 1955, Virology is a broad and inclusive journal that welcomes submissions on all aspects of virology including plant, animal, microbial and human viruses. The journal publishes basic research as well as pre-clinical and clinical studies of vaccines, anti-viral drugs and their development, anti-viral therapies, and computational studies of virus infections. Any submission that is of broad interest to the community of virologists/vaccinologists and reporting scientifically accurate and valuable research will be considered for publication, including negative findings and multidisciplinary work.Virology is open to reviews, research manuscripts, short communication, registered reports as well as follow-up manuscripts.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


