D2EHPA分离甘氨酸基培养基中的镉和镍
IF 5
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
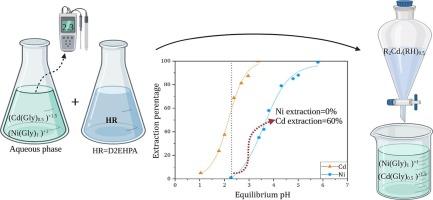
我们之前的研究表明,甘氨酸基浸出剂可以有效地从废镍镉电池中浸出镉(Cd)和镍(Ni)。本文首次报道了利用双-2-乙基己基磷酸(D2EHPA)萃取剂从甘氨酸基水溶液中分离镉(Cd)和镍(Ni)的工艺过程。考察了平衡pH、D2EHPA浓度、温度等相关参数的影响。当Ni和Cd浓度均为2.5 (g/L),有机水体积比为1时,经D2EHPA (0.6 M)萃取的甘氨酸溶液在25°C下的ΔpH0.5Ni-Cd(金属提取率达到50%时的平衡pH差)约为1.5;在相同条件下,pH = 4时,分离因子βCd/Ni值为555。基于甘氨酸介质中存在的水相的热力学模型、傅里叶变换红外光谱(FT-IR)和斜率分析方法,试图阐明Cd和Ni的萃取机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Separation of cadmium and nickel from glycine-based medium by D2EHPA
Our previous research demonstrates the efficient leachability of cadmium (Cd) and nickel (Ni) from the spent Ni-Cd batteries by using the glycine-based lixiviant. Here, for the first time, we report the separation process of cadmium (Cd) and nickel (Ni) from the glycine-based aqueous medium via bis-2-ethylhexyl phosphoric acid (D2EHPA) extractant. The effect of pertinent parameters including equilibrium pH, D2EHPA concentration, and temperature were examined. The (difference between the equilibrium pH at which 50 % extraction of metal occurs) for the glycine solution via D2EHPA (0.6 M) was approximately 1.5 at 25 °C when both Ni and Cd concentration is 2.5 (g/L) and the organic to aqueous volume ratio equals 1; moreover, the value of separation factor (βCd/Ni) was estimated 555 at pH = 4 under similar conditions. It was attempted to clarify the Cd and Ni extraction mechanisms based on the thermodynamic modeling of the aqueous species present in the glycine medium, Fourier Transform Infrared Spectroscopy (FT-IR), and slope analysis method.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


