黄芩苷纳米前药的制备及其对三阴性乳腺癌转移的抑制作用
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-08-18
DOI:10.1016/j.bioadv.2025.214464
引用次数: 0
摘要
背景三阴性乳腺癌(triple negative breast cancer, TNBC)由于缺乏特异性的治疗靶点且易转移,在临床治疗中面临很大挑战。天然成分黄芩苷能有效抑制TNBC的生长和转移;然而,它也有一些局限性,如靶向性差和副作用。纳米靶向给药系统可以通过促进药物积累和控制药物释放来提高药物疗效。目的trop -2跨膜糖蛋白在TNBC细胞中高表达,提示其可作为TNBC特异性活性靶向分子修饰纳米给药系统,克服TNBC的非特异性分布。基于肿瘤微环境中高浓度谷胱甘肽的特点,设计了氧化还原敏感纳米前药(Trop2-BA-ss-PPEP),实现药物的智能缓控释。方法对Trop2-BA-ss-PPEP的化学结构、稳定性、体外药物释放还原性、靶向性等进行表征。细胞实验和移植瘤模型验证了其抗肿瘤作用和生物安全性。结果strop2 - ba -ss- ppep在生理环境下稳定,并在还原条件下快速释放药物。实验表明,Trop2-BA-ss-PPEP显著促进了细胞摄取,增加了药物在肿瘤部位的积累和维持时间。增强了体内和体外对肿瘤转移的抑制作用,未见明显毒副作用。结论成功构建了trop2 - ba -ss- ppep。Trop2-BA-ss-PPEP的靶向能力、微环境反应性和抗肿瘤转移作用为TNBC治疗提供了新的策略,具有良好的应用和转化潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
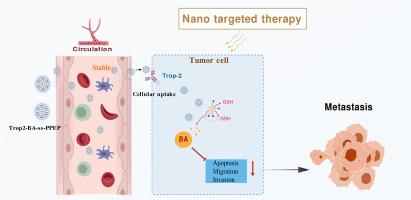
Preparation of baicalin nano prodrug and its effect on inhibiting metastasis of triple-negative breast cancer
Background
Triple-negative breast cancer (TNBC) faces great challenges in clinical treatment, owing to the lack of specific therapeutic targets and easy metastasis. The natural component baicalin can effectively inhibit the growth and metastasis of TNBC; however, it has some limitations, such as poor targeting and side effects. Nano targeted delivery systems can improve drug efficacy by enhancing drug accumulation and controlling drug release.
Objective
Trop-2 transmembrane glycoprotein expression is high in TNBC cells, suggesting that it can serve as a specific active targeting molecular-modified nano drug delivery system for TNBC to overcome non-specific distribution. Based on the characteristics of high-concentration glutathione in the tumor microenvironment, redox-sensitive nano-prodrugs (Trop2-BA-ss-PPEP) have been designed to achieve intelligent slow control and release of drugs.
Methods
The chemical structure of the Trop2-BA-ss-PPEP, and its stability, reductive response to drug release behavior, and targeting ability in vitro were characterized. Cell experiments and a transplanted tumor model verified the anti-tumor effect and biosafety.
Results
Trop2-BA-ss-PPEP was stable in a physiological environment and rapidly released the drug under reducing conditions. The experiments showed that Trop2-BA-ss-PPEP significantly promoted cellular uptake, and drug accumulation and maintenance time at the tumor site were increased. It enhanced the inhibitory effect on metastasis in vivo and in vitro, and no obvious toxicity or side effects were observed.
Conclusion
Trop2-BA-ss-PPEP was successfully constructed. The targeting ability, microenvironment responsiveness, and anti-tumor metastatic effects of Trop2-BA-ss-PPEP provide a new strategy for TNBC therapy, which has good application and transformation potential.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


