加速可持续纳米医学发展的数字化设计质量
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
药物输送系统、诊断和治疗都被纳米医学彻底改变,极大地改善了当代医疗保健。然而,传统的纳米药物开发依赖于大量的实验测试,这是昂贵的、耗时的,而且对环境有害。为了应对这些挑战,已经开发了数字设计质量(QbDD)概念和框架,集成了大量数据分析、人工智能(AI)、机器学习(ML)和计算建模等数字技术,以改变纳米颗粒设计。QbDD支持智能数字模拟和预测分析,以精确的生物物理化学特性改善纳米颗粒,从而提高批次可重复性,同时减少对资源密集型物理实验的依赖,降低成本,并最大限度地减少对环境的影响。这种数字基础设施不仅优化了纳米颗粒的设计和功效,而且确保了符合监管标准。本文综述了纳米信息学的基本原理及其与纳米医学中QbDD的集成,重点关注人工智能驱动的分子模拟和硅筛选,以预先选择纳米颗粒候选物,从而减少对劳动密集型实验验证的依赖。此外,它还讨论了纳米制药中的数字创新,包括实时监测系统和用于过程验证的数字双胞胎,强调了这些进步如何增强纳米颗粒合成,使其更高效、更具成本效益和可持续性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
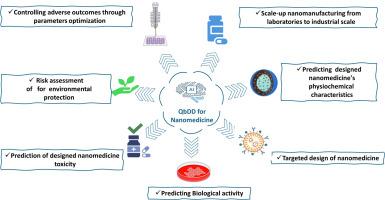
Quality by digital design for accelerated sustainable nanomedicine development
Drug delivery systems, diagnostics, and treatments are all being revolutionized by nanomedicine, greatly improving contemporary healthcare. However, traditional nanomedicine development relies on extensive experimental testing, which is costly, time-consuming, and environmentally harmful. To address these challenges, the Quality by Digital Design (QbDD) concept and framework have been developed, integrating digital technologies such as substantial data analytics, Artificial Intelligence (AI), Machine Learning (ML), and computational modeling to transform nanoparticle design. QbDD enables smart digital simulations and predictive analytics to improve nanoparticles with precise bio-physicochemical properties, thereby enhancing batch reproducibility while reducing reliance on resource-intensive physical experiments, lowering costs, and minimizing environmental impact. This digital infrastructure not only optimizes nanoparticle design and efficacy but also ensures compliance with regulatory standards. This review examines the fundamentals of nano-informatics and its integration with QbDD in nanomedicine, focusing on AI-powered molecular simulations and in silico screening to pre-select nanoparticle candidates, thereby reducing dependence on labor-intensive experimental validation. Furthermore, it discusses digital innovations in nanopharmaceuticals, including real-time monitoring systems and digital twins for process verification, highlighting how these advancements enhance nanoparticle synthesis, making it more efficient, cost-effective, and sustainable.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


