通过operando x射线成像实时洞察锂硫电池微孔噻吩聚合物
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
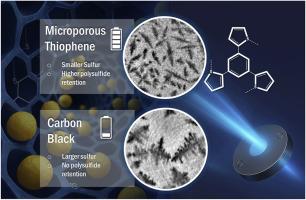
微孔噻吩聚合物(MTP-1)是一种通过环保方法合成的多孔有机聚合物(POP)。在MTP-1合成后,硫(S8)和生成的化合物MTP-1/S8被彻底表征。电化学测试表明,MTP-1/S8作为一个强大的正极,在100次循环后保持超过70%的容量。紫外可见光谱证实了该化合物吸收和捕获锂多硫化物(LiPSs)的能力。Operando电化学阻抗分析表明MTP-1/S8中的硫约束得到了改善,与整个研究中作为参考的炭黑/硫(CB/S8)控制电极相比,循环过程中电解质电阻的变化更小。相比之下,CB/S8表现出明显的电阻波动,表明多硫化物在电解质中的扩散更大,穿梭效应更强。Operando x射线照相实验提供了对硫形成和溶解过程的见解,与比容量值相关。在这两种体系中,硫颗粒的大小和分布有明显的不同。CB/S8表现出较少但较大的硫颗粒,表明在正极附近的多硫保留较低。相比之下,MTP-1/S8显示出更多的小硫颗粒,表明多硫保留更好,成核动力学增强。总体而言,绿色合成、先进表征以及原位和操作技术的使用强调了MTP-1/S8作为高性能硫宿主的潜力。这些发现有助于锂硫电池的发展,并提高对降解途径的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Real-time insights into microporous thiophene polymer for lithium-sulfur batteries via operando X-ray imaging
This study focuses on the Microporous Thiophene Polymer (MTP-1), a porous organic polymer (POP) synthesized through environmentally friendly methods. After MTP-1 synthesis, sulfur (S8) and the resulting compound, MTP-1/S8, is thoroughly characterized. Electrochemical tests show that MTP-1/S8 acts as a robust positive electrode, retaining over 70 % of its capacity after 100 cycles. UV–vis spectroscopy confirms the compound's ability to absorb and trap lithium polysulfides (LiPSs). Operando electrochemical impedance analysis reveals improved sulfur confinement in MTP-1/S8, evidenced by smaller variations in electrolyte resistance over cycling when compared to the Carbon Black/Sulfur (CB/S8) control electrode used as reference throughout the study. In contrast, CB/S8 exhibits significant resistance fluctuations, indicating greater polysulfide diffusion into the electrolyte and a stronger shuttle effect. Operando X-ray radiography experiments provide insights into the processes of sulfur formation and dissolution, correlating with specific capacity values. A distinct difference is observed in the size and distribution of sulfur particles between the two systems. The CB/S8 exhibits fewer but larger sulfur particles, suggesting lower polysulfide retention near the positive electrode. In contrast, MTP-1/S8 displays a higher number of smaller sulfur particles, indicating better polysulfide retention and enhanced nucleation kinetics. Overall, the green synthesis, advanced characterization, and use of in-situ and operando techniques emphasize MTP-1/S8 potential as a high-performance sulfur host. The findings contribute to lithium-sulfur battery development and improve the understanding of degradation pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


