离子液银协同催化环境CO2转化α-烷基烯环碳酸盐
IF 5.8
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
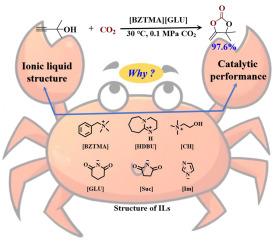
丙炔醇与CO2的羧基环化反应是一种原子经济的CO2利用方法,可以生成多功能的α-烷基烯环碳酸盐,作为药物合成和聚合物生产的关键中间体。尽管具有合成价值,但这种转化通常需要苛刻的反应条件,并且表现出令人不满意的效率。有必要开发先进的催化系统。研制了一系列戊二胺基离子液体,并对其与多种银共催化剂的协同作用进行了评价。[BZTMA][GLU]/Ag2CO3体系表现出优异的性能,在非常温和的条件下(6 mol% Ag2CO3, 30°C, 0.1 MPa CO2),产率达到97.6%。综合构效关系研究揭示了控制催化效率的两个关键参数:离子相互作用在−5.20 ~−11.26 kcal mol−1范围内,每mol离子液体的CO2吸收率超过1.33 mol。结合多核1H NMR、13C NMR和密度泛函理论(DFT)计算的机理研究阐明了协同活化途径。离子液体通过氢键促进底物激活,而银配合物优先激活炔部分,通过精心安排的催化循环共同实现有效的环化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synergistic ionic liquid-silver catalysis for ambient CO2 conversion to α-alkylidene cyclic carbonates
The carboxylative cyclization of propargylic alcohols with CO2 constitutes an atom-economical approach for CO2 utilization, yielding versatile α-alkylidene cyclic carbonates that serve as key intermediates in pharmaceutical synthesis and polymer production. Despite its synthetic value, this transformation typically requires harsh reaction conditions and exhibits unsatisfied efficiency. It is necessary to develop the advanced catalytic systems. A series of glutarimide-based ionic liquids were developed and their synergistic effects with various silver co-catalysts were evaluated. The [BZTMA][GLU]/Ag2CO3 system demonstrated exceptional performance, achieving 97.6 % yield under remarkably mild conditions (6 mol% Ag2CO3, 30 °C, 0.1 MPa CO2). Comprehensive structure-activity relationship studies revealed two critical parameters governing catalytic efficiency: the ionic interaction falls within the range of −5.20 to −11.26 kcal mol−1 and the CO2 absorption exceeds 1.33 mol for per mol of ionic liquid. Mechanistic investigations combining multinuclear 1H NMR, 13C NMR and density functional theory (DFT) calculations elucidated the cooperative activation pathway. The ionic liquid facilitates substrate activation through hydrogen bonding, while the silver complex preferentially activates the alkyne moiety, collectively enabling efficient cyclization through a well-orchestrated catalytic cycle.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


