多模态成像透明质酸胶束增强轻度光热治疗三阴性乳腺癌
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-08-12
DOI:10.1016/j.bioadv.2025.214460
引用次数: 0
摘要
轻度光热疗法(PTT)因其对癌细胞的选择性靶向和治疗的温和性而受到广泛关注。然而,其疗效受到肿瘤异质性和癌细胞对治疗的耐药性的限制。耐药癌症干细胞(CSCs)的自我更新能力和癌细胞中上皮-间质转化(EMT)的激活在很大程度上促进了残留肿瘤的复发和转移。在这项研究中,我们开发了一种具有cd44靶向能力的自组装胶束(HA-ADH@IR808),旨在提高轻度PTT治疗三阴性乳腺癌(TNBC)的效果。HA-ADH@IR808胶束内的肼基团在4.4 ppm和5.4 ppm时产生强烈的化学交换饱和转移(CEST)信号,从而实现光敏剂的精确肿瘤内定位。多模态成像通过精确定位光敏剂和实时监测治疗温度来提高轻度PTT的疗效,从而最大限度地减少副作用。体内实验显示,靶向CD44的轻度PTT可显著抑制癌细胞增殖,表明选择性消融CD44+细胞(主要是csc)可降低肿瘤生长和转移潜力。此外,我们的研究发现,低温光热处理诱导肿瘤细胞外基质(ECM)中胶原I的降解,从而导致与EMT途径相关的蛋白表达减少。总的来说,本研究为轻度光热治疗胶束的设计提供了新的见解,并在体内可视化和治疗监测方面取得了进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
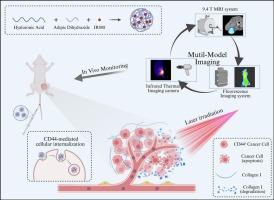
A multi-modal imaging hyaluronic acid micelle for enhanced mild photothermal therapy of triple-negative breast cancer
Mild photothermal therapy (PTT) for cancer treatment has gained significant attention due to its selective targeting of cancer cells and the mildness of the treatment. However, its efficacy is limited by tumor heterogeneity and the resistance of cancer cells to treatment. The self-renewal capacity of therapy-resistant cancer stem cells (CSCs) and the activation of epithelial-mesenchymal transition (EMT) in cancer cells largely contribute to the recurrence and metastasis of residual tumors. In this study, we developed a self-assembling micelle (HA-ADH@IR808) with CD44-targeting capabilities, designed to enhance the performance of mild PTT in the treatment of triple-negative breast cancer (TNBC). The hydrazide group within the HA-ADH@IR808 micelles generates a strong chemical exchange saturation transfer (CEST) signal at 4.4 ppm and 5.4 ppm, enabling precise intratumoral mapping of the photosensitizer. Multi-modal imaging enhances the efficacy of mild PTT by enabling accurate localization of the photosensitizer and real-time monitoring of treatment temperature, thereby minimizing side effects. In vivo experiments revealed that CD44-targeted mild PTT significantly inhibits cancer cell proliferation, suggesting that the selective ablation of CD44+ cells—predominantly CSCs—results in reduced tumor growth and metastatic potential. In addition, our study found that low-temperature photothermal treatment induced the degradation of collagen I in the tumor extracellular matrix (ECM), which subsequently led to a reduction in the expression of proteins associated with the EMT pathway. Overall, this study provides new insights into the design of mild photothermal therapeutic micelles, as well as advancements in in vivo visualization and treatment monitoring.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


