微波辐照沉淀聚合法制备依诺肝素印迹聚合物及其性能评价
IF 5.1
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
本研究制备了一系列依诺肝素印迹聚合物,并对其分子识别性能进行了评价。以衣康酸(ITA)为功能单体,N, N ' -亚甲基双丙烯酰胺(MBAA)为交联剂,以水为溶剂,合成了分子印迹聚合物(MIPs)和非印迹聚合物(NIPs)。采用微波辐照和常规搅拌两种方法,采用沉淀聚合法制备mip。聚合物形成后,对mip进行了表征,并对其吸附能力进行了评价。在最后阶段,利用MIPs开发了在生物基质(特别是血浆)中制备依诺肝素样品的方法。MIPM的吸附量为43.47±0.40 mg g−1,高于MIPS的40.77±0.75 mg g−1。MIPM和MIPS的印迹因子分别为1.21和0.71。吸附动力学评价表明,合成的mip均符合准二级动力学模型,相关系数接近于1。此外,吸附等温线分析表明,所有MIPs都符合Freundlich等温线模型。BET分析显示,MIPM的表面积最大,为20.186 m2/g。选择性实验表明,与MIPS相比,MIPM对依诺肝素具有更高的选择性。应用结果表明,MIPM比MIPS更有效地从血浆基质中提取依诺肝素。以20 mg MIPM为吸附剂,从血浆中提取依诺肝素,回收率为100.31±0.21%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
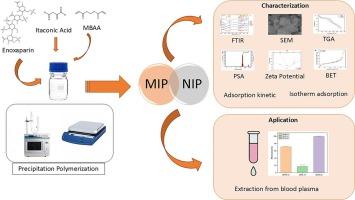
Preparation and evaluation of enoxaparin-imprinted polymer via precipitation polymerization using microwave irradiation and conventional stirring methods
In this investigation, a series of enoxaparin-imprinted polymers were prepared, and their molecular recognition properties were assessed. Itaconic acid (ITA) was utilized as the functional monomer, while N, N′-methylenebisacrylamide (MBAA) functioned as the cross-linker, with water serving as the solvent in the synthesis of both molecularly imprinted polymers (MIPs) and non-imprinted polymers (NIPs). The MIPs were produced through precipitation polymerization, employing both microwave irradiation and conventional stirring methods. Following polymer formation, the MIPs were characterized and their adsorption capacities were evaluated. In the final stage, a method for enoxaparin sample preparation in a biological matrix —specifically, blood plasma—was developed using the MIPs. The adsorption capacity of MIPM was 43.47 ± 0.40 mg g−1, exceeding that of MIPS, which was 40.77 ± 0.75 mg g−1. The imprinting factors (IF) for MIPM and MIPS were 1.21 and 0.71, respectively. The evaluation of adsorption kinetics revealed that all synthesized MIPs adhered to the pseudo-second-order kinetic model, as indicated by correlation coefficients approaching one. Additionally, the adsorption isotherm analysis showed that all MIPs were consistent with the Freundlich isotherm model. BET analysis showed that MIPM exhibited the largest surface area, measuring 20.186 m2/g. The selectivity test demonstrated that MIPM possessed greater selectivity for enoxaparin compared to MIPS. The application results demonstrated that MIPM was more effective than MIPS in extracting enoxaparin from the blood plasma matrix. Using 20 mg of MIPM as the sorbent, enoxaparin was extracted from blood plasma with a recovery rate of 100.31 ± 0.21 %.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


