组胺1受体和反向模式Na+/Ca2+交换驱动细胞外Na+依赖的细胞内Ca2+振荡在人脑血管内皮细胞
IF 4
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
摘要
脑血管内皮细胞是血脑屏障(BBB)的核心组成部分,在调节突触周围局部离子微环境中起着关键作用。因此,在强烈的神经元放电或病理状态(如扩张性抑郁)时,脑血管内皮细胞的胞外钾和钠离子浓度会发生剧烈变化。在此,我们评估了细胞外钠浓度([Na+]o)的降低触发hCMEC/D3细胞系中复杂Ca2+信号的机制,hCMEC/D3细胞系是人类血脑屏障最广泛的模型。我们证明降低[Na+]o引起多种Ca2+信号,包括细胞内Ca2+浓度([Ca2+]i)的单调增加和[Ca2+]i的重复振荡,这是由反向模式Na+/Ca2+交换器和组胺1受体(H1R)触发的。此外,我们提供了第一个证据,证明H1R可能在将[Na+]o的减少转化为磷脂酶C的激活和随后的肌醇三磷酸(InsP3)的产生中发挥关键作用,从而诱导内质网(ER)上的InsP3受体的节律性激活和ER Ca2+池的逐渐耗尽。内质网Ca2+浓度的下降导致快速存储操作的Ca2+进入激活,通过快速重新填充内质网Ca2+存储来维持细胞内Ca2+振荡。由[Na+]o的减少引起的内皮Ca2+振荡可能导致一氧化氮释放。因此,这些发现揭示了Gq蛋白偶联受体(gqpcr)在人类神经血管单元中塑造内皮Ca2+信号和Ca2+依赖事件的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
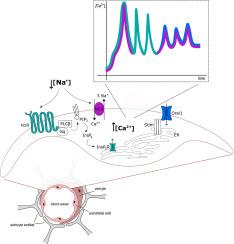
Histamine 1 receptors and reverse-mode Na+/Ca2+ exchanger drive extracellular Na+-dependent intracellular Ca2+ oscillations in human cerebrovascular endothelial cells
Cerebrovascular endothelial cells represent the core component of the blood-brain barrier, (BBB) which plays a critical role in regulating the local ionic microenvironment around the synapses. Therefore, cerebrovascular endothelial cells experience dramatic changes in the extracellular concentrations of potassium and sodium ions during intense neuronal firing or pathological conditions, such as spreading depression.
Herein, we assessed the mechanisms by which a reduction in extracellular sodium concentration ([Na+]o) triggers complex Ca2+ signals in the hCMEC/D3 cell line, which is the most widespread model of human BBB.
We demonstrate that lowering the [Na+]o elicits a variety of Ca2+ signals, including monotonic increases in intracellular Ca2+ concentration ([Ca2+]i) and repetitive oscillations in [Ca2+]i, which are triggered by the reverse-mode Na+/Ca2+ exchanger and histamine 1 receptor (H1R). Furthermore, we provide the first evidence that H1R may play a critical role in translating a reduction in [Na+]o into the activation of phospholipase C and following production of inositol triphosphate (InsP3), thereby inducing the rhythmic activation of InsP3 receptors on the endoplasmic reticulum (ER) and progressive depletion of the ER Ca2+ pool. The fall in the ER Ca2+ concentration leads to quick Store-Operated Ca2+ Entry activation, which maintains the intracellular Ca2+ oscillations by rapidly refilling the ER Ca2+ store. The endothelial Ca2+ oscillations induced by the reduction in [Na+]o may then lead to nitric oxide release.
These findings, therefore, shed novel light on the mechanisms whereby Gq protein coupled receptors (GqPCRs) can shape endothelial Ca2+ signaling and Ca2+-dependent events at the human neurovascular unit.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Cell calcium
生物-细胞生物学
CiteScore
8.70
自引率
5.00%
发文量
115
审稿时长
35 days
期刊介绍:
Cell Calcium covers the field of calcium metabolism and signalling in living systems, from aspects including inorganic chemistry, physiology, molecular biology and pathology. Topic themes include:
Roles of calcium in regulating cellular events such as apoptosis, necrosis and organelle remodelling
Influence of calcium regulation in affecting health and disease outcomes
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


