研究碱基化学对掺杂效率和结晶性的影响
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
氟掺杂氧化锡(FTO)是一种应用广泛的透明导电氧化物,但由于掺杂效率低,通过水热法合成其仍然具有挑战性。通过酸或碱调节反应介质的pH值,通过影响锡前驱体的水解速率和氟源的溶解度,对掺杂效率起着至关重要的作用。本研究系统地研究了pH对氢氧化铵(NH4OH)和四甲基氢氧化铵(TMAH)两种碱基调节剂水热合成的f掺杂SnO2纳米颗粒掺杂效率和结晶度的影响。结果表明,pH值直接影响结晶尺寸和氟的掺入。成功获得了具有四边形金红石相的SnO2纳米颗粒,其平均晶粒尺寸从4.51 nm (pH ~ 0.6,未经调整)减小到3.65 nm (NH4OH, pH 7)和3.45 nm (TMAH, pH 7),这是由于较高pH下水解和成核的增强。虽然降低质子浓度(pH > 3)促进了Sn-F相互作用,但过量的OH -和碱衍生阳离子会阻碍氟的扩散。在平衡水解动力学和阳离子干扰的条件下,F/Sn比分别为0.61 (NH4OH, pH 3)和0.36 (TMAH, pH 2)。元素分析和光谱分析进一步证实,氟通过O-to-F取代被成功地纳入SnO2晶格。这些发现强调了精确的pH控制在调整f掺杂SnO2的结构和化学性质方面的重要性,增强了其未来光电应用的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
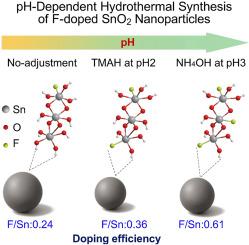
Investigating the role of base chemistry in doping efficiency and crystallinity of F-doped SnO2 nanoparticles
Fluorine-doped tin oxide (FTO) is a widely used transparent conductive oxide, yet its synthesis through hydrothermal methods remains challenging due to low dopant efficiency. The pH of the reaction medium, adjusted by acids or bases, plays a crucial role in determining doping efficiency by influencing the hydrolysis rate of Sn precursors and the solubility of fluorine sources. This study systematically investigated the effect of pH on doping efficiency and crystallinity in F-doped SnO2 nanoparticles synthesised hydrothermally using two base adjusters: ammonium hydroxide (NH4OH) and tetramethylammonium hydroxide (TMAH). The results showed that pH directly affected both crystallite size and fluorine incorporation. Fine crystalline SnO2 nanoparticles with a tetragonal rutile phase were successfully obtained, with average crystallite sizes decreasing from 4.51 nm (pH ∼0.6, unadjusted) to 3.65 nm (NH4OH, pH 7) and 3.45 nm (TMAH, pH 7), driven by enhanced hydrolysis and nucleation at higher pH. While decreasing proton concentration (at pH > 3) promotes Sn–F interaction, excessive OH− and base-derived cations can hinder fluorine diffusion. The optimal F/Sn ratios of 0.61 (NH4OH, pH 3) and 0.36 (TMAH, pH 2) were achieved under conditions that balance hydrolysis kinetics and cation interference. Elemental and spectroscopic analysis further confirmed that fluorine was successfully incorporated into the SnO2 lattice via O-to-F substitution. These findings emphasise the importance of precise pH control in tailoring the structural and chemical properties of F-doped SnO2, reinforcing its potential for future optoelectronic applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


