弗里德林诱导口腔癌细胞凋亡:来自体外和计算机研究的见解
Q1 Medicine
Journal of oral biology and craniofacial research
Pub Date : 2025-08-20
DOI:10.1016/j.jobcr.2025.08.013
引用次数: 0
摘要
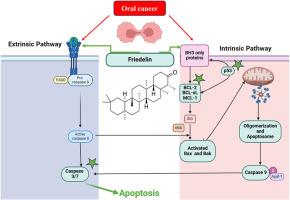
背景:由于其高发病率和死亡率,口腔癌是世界上一个主要的健康问题。失调的细胞凋亡,参与肿瘤进展和治疗抵抗。弗里德林是一种天然的三萜,已被证明具有调节细胞凋亡途径的潜力。本研究探讨了弗里德林的治疗作用,特别是其与凋亡蛋白的相互作用,对KB口腔癌细胞的细胞毒性作用,以及通过内在信号通路诱导细胞凋亡的能力。材料与方法选取CTD和GeneCards鉴定的freidelin和口腔癌相关靶基因,构建相互作用网络,并应用于STITCH数据库进行分析。采用分子对接法测定关键蛋白对Friedelin的凋亡结合亲和力。通过MTT、形态学分析和Annexin V-FITC流式细胞术等体外分析来获得弗里德林的细胞毒性潜能。通过基因表达分析证实了弗里德林对细胞凋亡的调控作用。结果网络分析确定了Friedelin与凋亡调节因子的关键相互作用。分子对接显示出较强的结合亲和力,特别是与Bax(−8.3 kcal/mol)和Bcl2(−8.0 kcal/mol)的结合。细胞毒性试验显示剂量和时间依赖效应。基因表达分析证实Bax、Caspase-3和TP53上调,Bcl2下调,表明激活了内在凋亡通路。结论弗里德林通过调节细胞凋亡信号通路,诱导细胞凋亡,是一种具有治疗口腔癌潜力的抗癌药物。未来的研究应在体内验证和临床翻译弗里德林作为一种新的候选治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Friedelin induces apoptosis in oral cancer: Insights from in vitro and in silico studies
Background
Oral cancer is a major health issue in the world because of its high morbidity and mortality rates. Dysregulated apoptosis, is involved in tumor progression and treatment resistance. Friedelin, a natural triterpenoid, has been shown to have potential to modulate apoptosis pathways. This study investigates the therapeutic effects of Friedelin, particularly on its interactions with apoptotic proteins, cytotoxic effects on KB oral cancer cells, and ability to induce apoptosis through intrinsic signaling pathways.
Materials and methods
Interaction networks were constructed by taking target genes related to Friedelin and oral cancer identified by CTD and GeneCards, and applying them in the STITCH database for analysis. Apoptotic binding affinity of key proteins, towards Friedelin was determined using molecular docking. The cytotoxic potential of Friedelin was accessed by performing in vitro assays such as MTT, morphology analysis, and Annexin V-FITC flow cytometry. The regulation of Friedelin on apoptosis was validated through gene expression analysis.
Results
Network analysis identified Friedelin's critical interactions with apoptotic regulators. Molecular docking revealed strong binding affinities, particularly with Bax (−8.3 kcal/mol) and Bcl2 (−8.0 kcal/mol). Cytotoxicity assays showed dose- and time-dependent effects. Gene expression analysis confirmed upregulation of Bax, Caspase-3, and TP53, and downregulation of Bcl2, demonstrating activation of intrinsic apoptotic pathways.
Conclusion
Friedelin is an anticancer agent that has great potential for oral cancer treatment by modulating apoptotic signaling pathways and inducing apoptosis. Future studies should be conducted on the in vivo validation and clinical translation of Friedelin as a novel therapeutic candidate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of oral biology and craniofacial research
Medicine-Otorhinolaryngology
CiteScore
4.90
自引率
0.00%
发文量
133
审稿时长
167 days
期刊介绍:
Journal of Oral Biology and Craniofacial Research (JOBCR)is the official journal of the Craniofacial Research Foundation (CRF). The journal aims to provide a common platform for both clinical and translational research and to promote interdisciplinary sciences in craniofacial region. JOBCR publishes content that includes diseases, injuries and defects in the head, neck, face, jaws and the hard and soft tissues of the mouth and jaws and face region; diagnosis and medical management of diseases specific to the orofacial tissues and of oral manifestations of systemic diseases; studies on identifying populations at risk of oral disease or in need of specific care, and comparing regional, environmental, social, and access similarities and differences in dental care between populations; diseases of the mouth and related structures like salivary glands, temporomandibular joints, facial muscles and perioral skin; biomedical engineering, tissue engineering and stem cells. The journal publishes reviews, commentaries, peer-reviewed original research articles, short communication, and case reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


